SOPHIA ANTIPOLIS, France – January 22, 2024 │ The quarterly report for the Q4 2023 Therapeutic mRNA patent monitor is now available! This report covers all aspects of mRNA design, delivery, manufacturing, storing, mRNA-based vaccines, and mRNA-based therapeutics. What’s new this quarter?
Q4 2023 IP Activity overview
The IP activity during Q4 2023 is consistent with the data observed in previous quarters. We can see significant IP activity from leaders in the field, such as MODERNA and BIONTECH, as well as from certain American universities such as JOHNS HOPKINS UNIVERSITY and the UNIVERSITY OF PENNSYLVANIA. It is also worth noting the significant number of patents granted to SANOFI in the last quarter, including some that were filed by TRANSLATE BIO. Additionally, two broad patent families related to alphaviral replicon particles from ALPHAVAX have expired.
IP activity during Q4 2023:
- 159 new patent applications published, 13 filed by BIONTECH, 13 filed by MODERNA, 5 filed by JOHN HOPKINS UNIVERSITY and 5 filed by PENN university;
- 33 newly granted patents (US/EP/JP/KR), 6 owned by SANOFI (4 filed by Translate Bio), 5 owned by MODERNA, 2 owned by JANSSEN and 2 owned by YALE UNIVERSITY;
- 20 abandoned or expired patents, mainly expired ALPHAVAX’s patents.
This IP activity covers mRNA design, delivery, manufacturing, storing, mRNA-based vaccines, and mRNA-based therapeutics. All of these segments are analyzed within the IP monitoring activity.
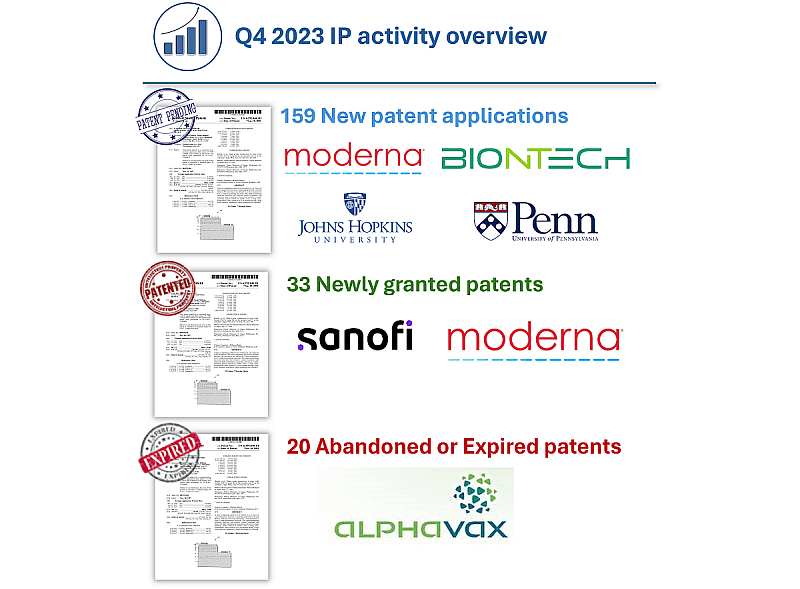
Figure 1: Q4 2023 IP Activity overview
Leaders for each legal status are highlighted. ©KnowMade.
Q4 2023 Litigation overview
Q4 2023 was marked by several new litigations procedures that might affect the whole therapeutic mRNA field, see figure 2. Indeed, ACUITAS filed a complaint against CUREVAC SE seeking inventorship in the US, see our previous analysis for more details on its potential impact on COVID-19 vaccine’s ecosystem. In addition, SANOFI filed two oppositions to two GSK’s patents related to mRNA delivery at the European Patent Office, while BIONTECH filed two oppositions to two CUREVAC’s patents related to mRNA synthesis and structure for enhanced protein expression. These litigations are detailed in KNOWMADE’s monitoring activity. In a dynamic technological field such as therapeutics mRNA, IP right enforcement is an important aspect to follow to get a deep understanding of the players and ecosystem dynamics. For example, as detailed in a previous analysis, another important litigation was observed in 2023 and is the subject of a previous analysis: ALNYLAM and its third set of lawsuit against MODERNA and PFIZER.
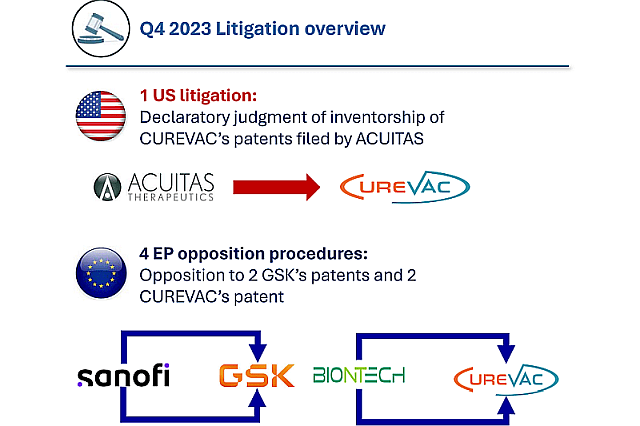
Figure 2: Q4 2023 Litigations Activity overview
©KnowMade.
Q4 2023 The closure of a dynamic year
This last quarter confirms all the tendencies observed throughout 2023, including a strong focus on Delivery dynamics, particularly with LNPs, mRNA modifications related to stability, immunogenicity, and efficiency, and strengthened technologies based on SA RNA. These developments have been detailed in KNOWMADE’s previous press releases:
- Q1 2023, with an overview of most dynamic segments;
- Q2, 2023, with a focus on LNP and Self-Amplifying mRNA segments; and
- Q3 2023, with a focus on mRNA modifications that ended up with a Nobel prize.
The patent monitoring activity also allows a monitoring on therapeutic tendencies as information on main IP players (established or newcomers), see our last Insight “A look back at mRNA patenting activity for 2023” for more information on 2023’s IP activity.
If you require additional information, please reach us at contact@knowmade.fr or with our contact form.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About the author
Elodie Bovier, PhD., works at KnowMade as a Patent Analyst in the field of Biotechnology and Life Sciences. She holds a PhD in genetic and molecular biology from the Paris Sud University. She also holds the Industrial Property International Studies Diploma (in Patent and Trademark & Design Law) from the CEIPI (Strasbourg, France).
About KnowMade
KnowMade is a technology intelligence and IP strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patent information and scientific literature into actionable insights, providing high added value reports for decision makers working in R&D, innovation strategy, intellectual property, and marketing. Our experts provide prior art search, patent landscape analysis, freedom-to-operate analysis, IP due diligence, and monitoring services.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Sensors, Semiconductor Packaging, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
