Monitoring litigations in the therapeutic mRNA field
SOPHIA ANTIPOLIS, France – June 06, 2023 │ With the COVID-19 crisis, mRNA technologies have become key for numerous players. After the success of mRNA vaccine and the related hype, players are looking to settle their activity and strengthen their position. With this new phase comes litigations. Moderna and Pfizer are already subject to attacks, but many others will follow. To keep an eye on and get the results of the ongoing and new US and EP litigations, KnowMade is dedicating a full section in its new mRNA monitor. The quarterly report for the Q2 2023 Therapeutic mRNA Patent Monitor will be released next July. This monitoring service covers all technological aspects of therapeutic mRNA, as well as players’ dynamics by following IP dynamics, patent transfers, and litigations. Let’s focus on monitoring litigation in the mRNA therapeutic area.
Patent litigations: A dynamic to follow
mRNA-related IP rights enforcement is an active aspect to follow to better understand players’ dynamics. The patent monitoring service from KnowMade allows to follow each quarter the new US patent litigations and EP oppositions. Various litigations are currently ongoing, such as the Moderna vs. Pfizer/BioNTech battle for patent infringement concerning the Comirnaty® RNA vaccine, filed last August (see our previous article Moderna vs Pfizer BioNTech – Let’s get inside the patent portfolio engaged in this battle for a detailed analysis).
Prior to patent infringement action in a national court players’ aggressiveness and “blocking” patents can be highlighted by monitoring opposition procedures at the European patent office (EPO). An opposition to a European patent is a post-grant procedure that can be filed within nine months following the mention of the patent’s grant in the European Patent Bulletin. Decisions of the EPO’s Opposition Division apply to all states designated in the European patent, and can result in one of three possible outcomes: the patent is maintained as granted, amended, or revoked. In the fourth quarter of 2022, in the field of mRNA therapeutics , eight opposition procedures were filed at the EPO, five of which were against Moderna’s EP patents. The main opponents were Sanofi (against Moderna, GSK, and Curevac patents) and BioNTech (against Moderna and Curevac patents); this is illustrated in the figure below. At the same time, no US litigation was filed this quarter.
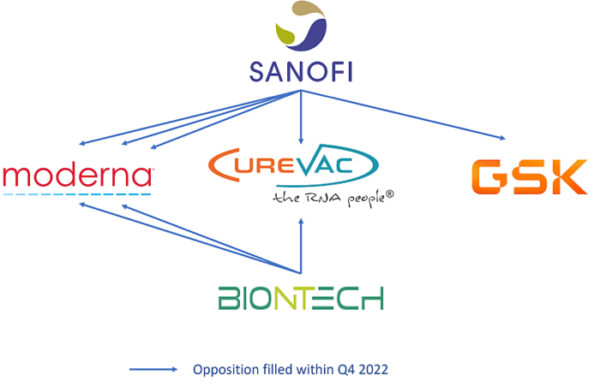
Figure 1: Schematic representation of EP opposition procedures filed within Q4 2022
(From KnowMade’s Monitoring reports Q4 2022).
During the same period, three decisions of the Opposition Division, all related to Curevac’s portfolio, were released (see table below). Two of these decisions are currently being appealed while the last decision resulted in the revocation of Curevac’s European Patent EP3319622, related to an in vitro method to produce an mRNA composition comprising mRNA encoding different variants of a peptide or protein.
| Assignee | Patent number | Title | Opponent(s) | Opposition outcome (Notification Date) |
Link to EPO Register |
| CUREVAC | EP3319622 | Method for producing RNA molecule compositions | BIONTECH | EP REVOKED (15/12/2022) |
Link |
| CUREVAC | EP3292873 | Combination of vaccination and inhibition of the pd-1 pathway | MERCK BIONTECH ETHERNA Pfizer STRAW MAN STRAW MAN |
EP REVOKED (22/12/2022) APPEAL FILED (23/01/2023) |
Link |
| CUREVAC | EP3062798 | Modified RNA with decreased immunostimulatory properties | STRAW MAN STRAW MAN |
REJECTION OF THE OPPOSITION (10/11/2022) APPEAL FILED (21/12/2022) |
Link |
Table 1: Q4 2022 New decisions of the opposition division
Note: STRAW MAN indicates opposition filed by companies acting as a front for other parties to conceal their identities (From KnowMade’s Monitoring reports Q4 2022).
The IP battle against Moderna & Pfizer continues in Q2 2023
Incoming quarterly report (Q2 2023, released in July 2023) will overview US and EP litigations in the therapeutic mRNA field, among them a LNPs related patent battle will be detailed. On May 26, 2023, Alnylam filed its third set of lawsuits against Moderna (Case No. 1:23-cv-00580) and Pfizer (Case No. 1:23-cv-00578) in Delaware Federal Court, claiming that the companies’ COVID-19 vaccines infringe its patents. The alleged patents are US Patent Nos. 11,590,229 (in actions against Pfizer and Moderna), 11,633,479 (in actions against Pfizer and Moderna), 11,633,480 (in actions against Pfizer and Moderna), 11,612,657 (in action against Pfizer). All these four patents are continuations of the same application No. 17/302,311 filled on April 29, 2021 (now Granted patent No. 11,246,933) and were recently granted (Feb. 28, 2023; Apr. 25, 2023; Apr. 25, 2023 and Mar. 28, 2023, respectively).
According to Alnylam’s complaints, Moderna’s infringing lipid particle is SM-102 and Pfizer’s infringing lipid particle is ALC-0315. Both are lipid particles that are part of the delivery system (LNP) for the COVID-19 vaccine (SpikevaxTM and ComirnatyTM). In both cases, Alnylam is seeking “reasonable royalty” for the alleged damages.
This new battle on LNP components ownership comes after Alnylam sued Moderna and Pfizer in March 2022, alleging that more than a decade ago they invented the delivery technology employed by both vaccines (these complaints are both based on the original US patent No. 11,246,933, and are still open).
LNP’s Power over mRNA Therapies
Therapeutic mRNA’s IP monitoring activity has already shown in the past two quarters that delivery is the most active technical segment and is highly dominated by LNP delivery systems, with more than 57 patent applications newly published in Q1 2023 (see Q1 2023 press release) and 41 in Q4 2022 (see Q4 2022 press release) related to such nanoparticles. This segment is also over-represented in core technologies for new startup firms. For example, in Q4 2022, four new companies with a strong focus on LNP were identified: Nanovation, THERNA Therapeutics, and West Gene Biopharma (see Q4 2022 press release).
This new battle of LNP components combined with the high patenting activity related to LNP as a delivery for therapeutic mRNA illustrates the importance of such a drug delivery system for drug delivery, and developing an efficient delivery system is the current ongoing challenge in this area.
Need more details or explanations: Ask our experts
Patenting and litigation activities related to delivery, and more specifically to LNP, highlight the importance of this delivery system for therapeutic mRNA and confirm the need for close monitoring of this topic.
The IP monitoring of mRNA therapeutics provides up-to-date data on patent activity and a litigation survey is a useful tool for anyone involved or interested in this therapeutic area. KnowMade’s service comes with privileged access to IP analysts for Q&A sessions and discussions regarding trends, analyses, specific patented technologies, or companies’ IP portfolios in the field of mRNA therapeutics.
If you need more information, reach us at contact@knowmade.fr or with our contact form.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About the author
Elodie Bovier, PhD., works at KnowMade as a Patent Analyst in the field of Biotechnology and Life Sciences. She holds a PhD in genetic and molecular biology from the Paris Sud University. She also holds the Industrial Property International Studies Diploma (in Patent and Trademark & Design Law) from the CEIPI (Strasbourg, France).
About KnowMade
KnowMade is a technology intelligence and IP strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patent information and scientific literature into actionable insights, providing high added value reports for decision makers working in R&D, innovation strategy, intellectual property, and marketing. Our experts provide prior art search, patent landscape analysis, freedom-to-operate analysis, IP due diligence, and monitoring services.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Sensors, Semiconductor Packaging, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
