SOPHIA ANTIPOLIS, France – November 27, 2023 │Acuitas Therapeutics filed a complaint on November 13, 2023, against CureVac SE seeking a declaratory judgment of inventorship regarding the US patents 11,241,493; 11,471,525; 11,576,966; and 11,596,686 (collectively referred to as Patent Family ‘493). According to the complaint (Case 3:23-cv-00764), the patents in question were wrongfully assigned to CureVac without acknowledging Acuitas’ scientists as co-inventors, despite their substantial contribution to the claimed inventions.
Acuitas Therapeutics claims CureVac’s patent inventorship
Acuitas asserts that it has been collaborating with CureVac since 2014 on the development of vaccines using Acuitas’ LNP technology, and in response to the COVID-19 pandemic, it offered to develop an mRNA vaccine against COVID-19 in cooperation with CureVac. According to the complaint, Acuitas’ scientists have provided essential expertise by inventing and formulating critical technologies for mRNA-LNP vaccines covered by the ‘493 Patent Family and CureVac filed the patent applications in secret, deliberately excluding Acuitas inventors. Acuitas, asserting that this failure to include them undermines their business model of partnership and intellectual property sharing, has therefore initiated a proceeding for the correction of named inventor (35S.S.C §256) of the ‘493 patent family. In this procedure, Acuitas is asking the court to order the correction of patents to include Drs. Michael Hope, Ying Tam, Paulo Lin, and Barbara Mui as co-inventors, and that the Director of the United States Patent Office issues a certificate of correction to that effect, which could allow Acuitas to obtain independent licenses.
ALC-0315, a key cationic lipid for lipid nanoparticles
The patent family ‘493 discloses a composition comprising a mRNA encoding a SARS-CoV-2 spike protein with K986P and V987P mutations and the cationic lipid ALC-0315 (named as formula III-3 in the patent). This cationic lipid (see Figure 1), developed by Acuitas, is disclosed within the US patent 11,648,324 belonging to Acuitas.
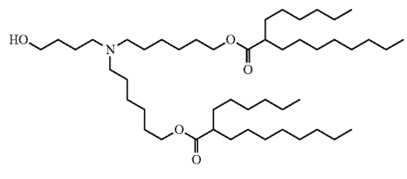
Figure 1: ALC-0315 developed by Acuitas Therapeutics
Acuitas already licenses its lipids to many partners, including CureVac, Pfizer and BioNTech. In particular, Acuitas’s lipids, including ALC-0315, and LNP technology are used to deliver the mRNA in Comirnaty®—the first FDA-approved COVID-19 vaccine developed by Pfizer & BioNTech (see Pfizer Press release).
BioNTech and Pfizer file a complaint against CureVac over patent infringement allegations
In 2022, BioNTech & Pfizer filed a complaint against CureVac for declaratory judgment of noninfringement of patents US 11,135,312, 11,149,278 and 11,241,493 (the one for which Acuitas is seeking inventorship) by the Comirnaty® vaccine (US case 2:23-cv-00222). This procedure can result from the court to prohibit CureVac from pursuing allegations of infringement against Comirnaty® based on its IP right protected by the US patent 11,135,312, 11,149,278 and 11,241,493. In May 2023, CureVac asserted counterclaims alleging that the COVID-19 vaccine developed by Pfizer and BioNTech infringes the ‘493 Patent Family, along with other patents. The case is still ongoing.
Regarding Patent ‘493, according to BioNTech and Pfizer’ complaint, Comirnaty® vaccine does not comprise a “composition comprising a mRNA comprising . . . at least one pharmaceutically acceptable carrier, wherein the mRNA is complexed or associated with lipid nanoparticles …” as required by all of the claims of the ‘493 patent to the extent understood. Therefore, the complaint suggests that the chemical relationships between lipids and mRNA are the main characteristics to determine if there is or not infringement.
In the description of patent ‘493, the terms “complexed” or “associated” are defined as the essentially stable combination of nucleic acid with one or more lipids into larger complexes or assemblies without covalent binding. The covalent or non-covalent nature of the association between mRNA and lipids could therefore be an important point for this complaint. Interestingly, Packer et al. in 2021 found that ionizable lipids in the LNP component can produce aldehyde impurities through oxidation and hydrolysis pathways and can form covalent adducts with mRNA, thus affecting mRNA biological activity. Indeed, PEG lipids are an important potential source of hydroperoxides, which are often unstable and whose degradation can lead to chain reactions and catalyze the degradation of ionizable lipids. Lipid degradation products can also react with mRNA, leading to the formation of covalent mRNA-lipid adducts. According to the authors, as many lipid-based nucleic acid formulations share common chemical functionalities, particularly those that use an ionizable amino-lipid, mechanisms identified in this study are broadly applicable. That might suggest the presence of covalent mRNA-lipid binding in mRNA-LNP therapeutics.
Potential lawsuits over patent infringement in the CureVac-BioNTech-Pfizer cases
Interestingly, the ‘493 Patent Family is at the center of several disputes involving CureVac, Acuitas, and BioNTech-Pfizer, which highlights its particularly strategic role (See Figure 2). Indeed, the financial stakes are significant. To date, Pfizer and BioNTech have supplied over 591 million doses of their COVID-19 vaccine for use in the United States, and Pfizer reported that the COVID-19 vaccine generated $7.8 billion in sales in the US in 2021 and $8.775 billion in 2022.
Acuitas Therapeutics’ lawsuit against CureVac over patent inventorship could potentially indicate the company’s intention to use the patent against BioNTech-Pfizer or other companies. It will thus be very interesting to follow both the outcome of Acuitas’ lawsuit against CureVac (inventorship) and BioNTech and Pfizer’s against CureVac (noninfringement) in order to understand how this situation may develop.
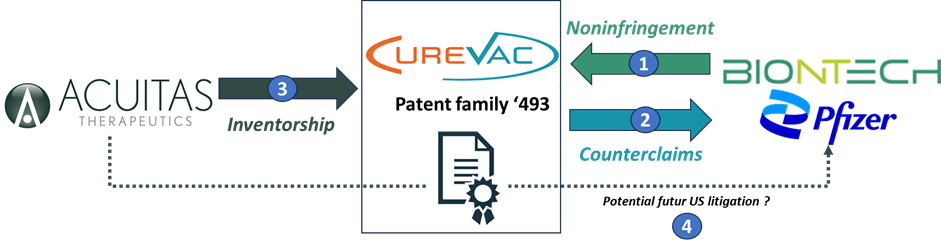
Figure 2: Summary of disputes mentioned around patent family ‘493.
Moreover, several other disputes related to mRNA vaccines, including Comirnaty, have recently been carried out, such as those by Alnylam described in the Knowmade article “Alnylam kicks off its third round in mRNA delivery battle“. Patenting and litigation related to delivery, specifically LNP, emphasizes the significance of this delivery system for therapeutic mRNA and underscores the necessity of closely monitoring this topic. To accomplish this, Knowmade tracks these disputes in the ‘Therapeutic mRNA Patent Monitor’.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
About the author
Brice Sagot, CEO and co-founder of KnowMade, leads the Biotechnology and Life Sciences department. He holds a PhD in Molecular Biology from the University of Nice SophiaAntipolis (France).
Elodie Bovier, PhD., works at KnowMade as a Patent Analyst in the field of Biotechnology and Life Sciences. She holds a PhD in genetic and molecular biology from the Paris Sud University. She also holds the Industrial Property International Studies Diploma (in Patent and Trademark & Design Law) from the CEIPI (Strasbourg, France).
About KnowMade
KnowMade is a technology intelligence and IP strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patent information and scientific literature into actionable insights, providing high added value reports for decision makers working in R&D, innovation strategy, intellectual property, and marketing. Our experts provide prior art search, patent landscape analysis, freedom-to-operate analysis, IP due diligence, and monitoring services.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Sensors, Semiconductor Packaging, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
