SOPHIA ANTIPOLIS, France – October 25, 2023 │ The quarterly report for the Q3 2023 Therapeutic mRNA patent monitor is now available! This report covers all aspects of mRNA design, delivery, manufacturing, storing, mRNA-based vaccines, and mRNA-based therapeutics. Let’s focus on an aspect of mRNA design that that ended up in a Nobel prize!
mRNA modification, a key for a Nobel prize
mRNA modification was essential for the development of mRNA therapeutics. Indeed, one of the main blocking points in developing an mRNA-based therapeutic approach was the unwanted inflammatory response combined with the mRNA translation inefficiency in cells and tissues. Unlocking this aspect was possible thanks to the discovery of K. Karikó and D. Weissman demonstrating that mRNA produced with modified nucleoside bases evades innate immune recognition and improves protein expression. This discovery earned them the Nobel prize in physiology or medicine this year (Nobel Prize Press release here). As illustrated in the figure below and detailed in the related scientific publication (Kariko et al., 2005), based-modified mRNA is less susceptible to induce an inflammatory response because such modifications suppress the potential of RNA to activate dendritic cells. As a result base-modified mRNAs are more efficient for encoded protein production in living tissues, as illustrated in the figure below from the related international patent application WO2007/024708.
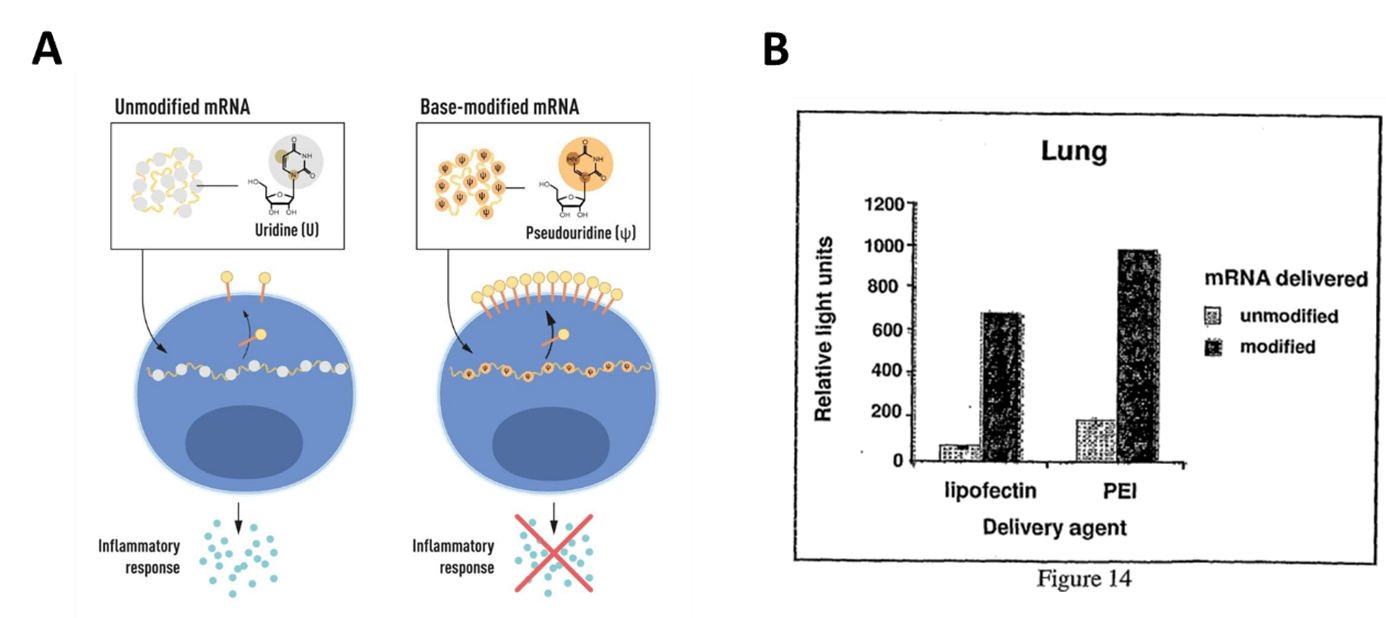
Figure 1: mRNA modification: a key for efficient mRNA therapeutics
A: mRNA contains four different bases, abbreviated A, U, G, and C. The Nobel Laureates discovered that the base-modified mRNA can be used to block activation of inflammatory reactions (secretion of signaling molecules) and increase protein production when the mRNA is delivered to cells. © The Nobel Committee for Physiology or Medicine. Ill. Mattias Karlén.
B: Expression of firefly luciferase following intratracheal delivery of encoding mRNA. mRNAs were complexed to lipofectin (or PEI, as noted) and animals were injected with 0.3 μg firefly luciferase-encoding mRNA with or without ψ modification, then sacrificed 3 hours later. Lungs were harvested and homogenized, and luciferase activity was measured in aliquots of the lysed organs. Figure 14 from WO2007/024708.
IP monitoring of mRNA modifications
mRNA modification developed for improving therapeutic mRNA efficiency can be located in three sub areas as illustrated in figure 2: the 5’ region (comprising the Cap structure and the 5’UTR), the coding region, and the 3’ region comprising the 3’UTR and the tail). Modified nucleosides as described in K. Karikó and D. Weissman work is not the only modification that can be made in the coding sequence to improve therapeutic mRNA efficiency. Indeed, nucleosides modification is often associated with codon optimization (e.g., humanized codon) to reduce mRNA immunogenicity and improve mRNA translation. In addition, mRNA optimization can be performed in the Cap structure, the 5’ and/or the 3’ UTR sequences. These modifications can result in enhanced mRNA stability by preventing its degradation, for example by using a lariat cap structure to enhance intracellular stability and translation as described in the patent application newly published this quarter KR10-2581491 from KAIST (Korea Advanced Institute Of Science & Technology), or in improved mRNA targeted expression as in MODERNA and CUREVAC recent strategy using specific miRNA silencing process to turn off therapeutic mRNA expression in undesired cells (CUREVAC) or to turn on therapeutic mRNA expression in desired cells (both of these strategies are detailed in KnowMade’s insight from March 2023 on MODERNA & CUREVAC).
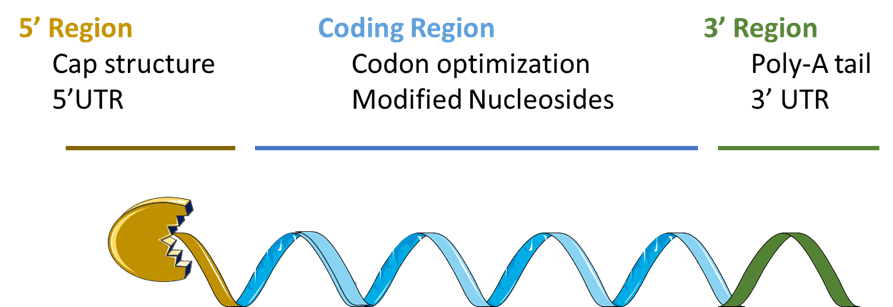
Figure 2: Schematic representation of mRNA
mRNA optimization can be made in the 5’ region including the Cap structure and 5’UTR, the coding region including optimized codon and/or modified nucleosides, and in the 3’ region including a 3’UTR and a poly-A tail. Each of these optimized regions acts on RNA stability and/or translation and/or immunogenicity. UTR: Untranslated Region. ©KnowMade.
It is very interesting to observe through the IP monitoring of mRNA therapeutics that this quarter, almost 21% of the newly published patent applications (US, EP, KR or JP) belong to the mRNA modification segment (see figure 3) while less than 10% were related to this segment last year. This segment comprises patent families related to mRNA modifications per se for general improvement (stability, immunogenicity, efficiency), for targeted delivery (e.g., switch on/switch off system), and/or for specific applications, such as switch on/switch off system for arteriosclerosis treatment (WO2023/164708 filed by the University of south Florida).
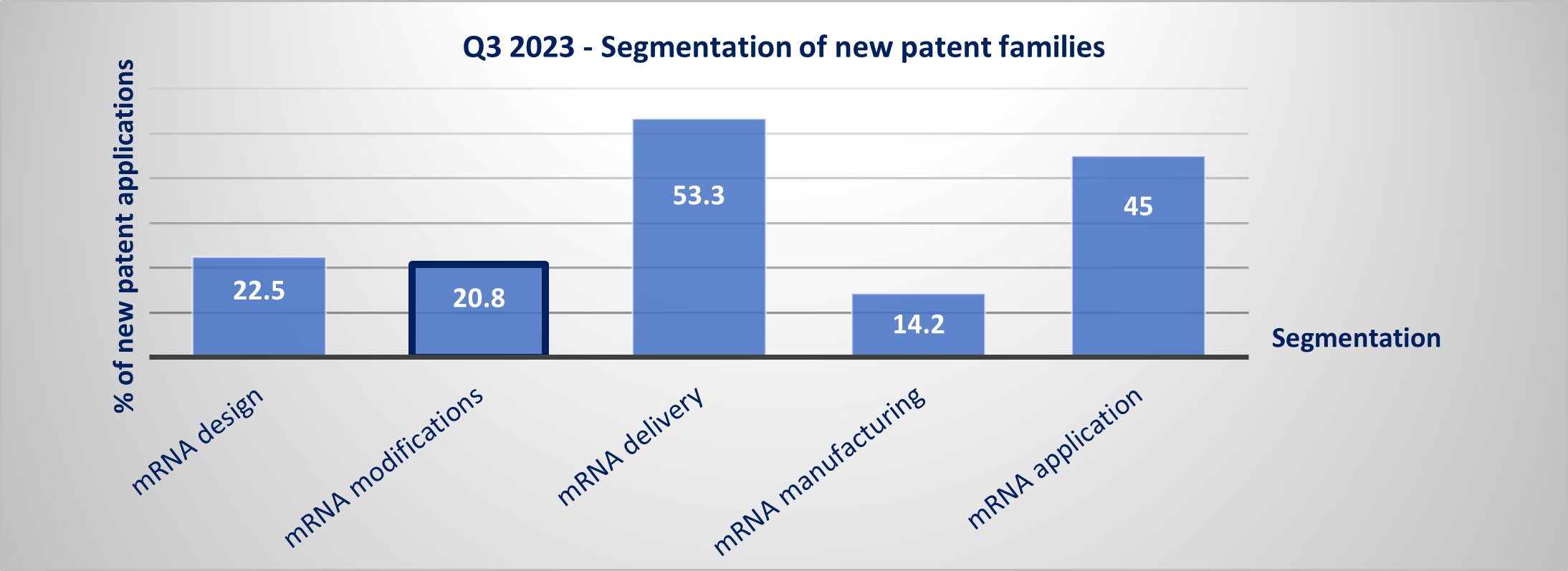
Figure 3: Segmentation of new patent families in Q3 2023
Newly published patent families (WO, EP, US, JP or KR) were manually segmented according to their technological features & applications. A patent family can belong to multiple segments. ©KnowMade.
The strategic topic of therapeutic mRNA efficiency relies on technological issues such as mRNA integrity, mRNA immunogenicity, and mRNA targeted delivery. As previously stated, enhancing these aspects can be achieved through mRNA modification. In addition, such challenge can also be overcome thanks to the choice and/or the development of optimal delivery vehicle for an mRNA therapeutic. It is worth to note that a strong IP dynamic was also observed in this technological segment as previously analyzed, see KnowMade’s Q2 2023 press release, Q1 2023 press release and Q4 2022 press release. The future might show a growing interest in two technological segments, mRNA modification and delivery, that are crucial for an efficient therapy based on mRNA.
The patent monitoring activity also allows a monitoring on therapeutic tendencies as information on main IP players (established or newcomers). If you need more information, reach us at contact@knowmade.fr or with our contact forms.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Sophia Antipolis, France
www.knowmade.com
About the author
Elodie Bovier, PhD., works at KnowMade as a Patent Analyst in the field of Biotechnology and Life Sciences. She holds a PhD in genetic and molecular biology from the Paris Sud University. She also holds the Industrial Property International Studies Diploma (in Patent and Trademark & Design Law) from the CEIPI (Strasbourg, France).
About KnowMade
KnowMade is a Technology Intelligence and IP Strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand their competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patent information and scientific literature into actionable insights, providing high added value reports for decision makers working in R&D, innovation strategy, intellectual property, and marketing. Our experts provide prior art search, patent landscape analysis, freedom-to-operate analysis, IP due diligence, and monitoring services.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Sensors, Semiconductor Packaging, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
