SOPHIA ANTIPOLIS, France – April 21, 2023 │ The quarterly report for the Q1 2023 Therapeutic mRNA patent monitor is now available! This report covers all aspects of mRNA design, delivery, manufacturing, storing, mRNA-based vaccines, and mRNA-based therapeutics. Let’s focus on current dynamics in mRNA design & delivery.
Q1 2023: Exploring the dynamic activity of therapeutic mRNA innovations
With 30% more new patent applications compared to the previous quarter, Q1 2023 confirms the dynamic activity in the therapeutic mRNA area. The fine-tuned segmentation of KnowMade’s monitoring activity allows for a deep understanding of technological sectors of current interest in therapeutic mRNA innovation. As previously observed, delivery is still the most active technical segment with more than 90 patent applications newly published this quarter. In addition, mRNA stability, immunogenicity, translation efficiency, etc. which are current challenges to improve therapeutic mRNA efficiency, represent more than 80 patent applications newly published. Therapeutic mRNA efficiency is in constant evolution, partly due to innovations in mRNA design and/or modifications. In Q1 2023, in addition to the >80 new patent applications related to mRNA design (conventional, self-amplifying, circular, or double-stranded), a significant number of new patent applications describes mRNA modifications mainly oriented towards translation efficiency or targeted delivery.
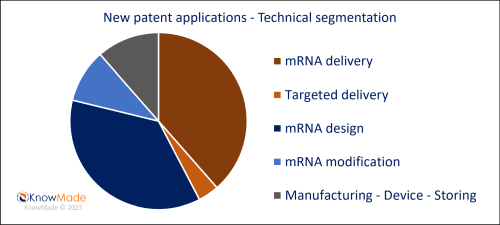
Figure 1: New patent applications in Q1 2023 – Technical segmentation
Newly published patent families (WO, EP, US, JP or KR) were manually segmented according to their technological features. A patent family can belong to multiple segments.
Focus on mRNA design & mRNA delivery
Among new patent applications related to mRNA design, a large portion describes non-conventional mRNA. All of these alternative designs show the same technical benefit of having a longer expression profile in cells. Of these new patent applications, 21% describe self-amplifying mRNA, confirming the potential of this design for vaccine approaches in oncology or to fight infectious diseases as recently suggested in KnowMade’s last report on saRNA Vaccine. Additionally, 7% describe circular mRNA from North American and Asian assignees (small companies or academics), and one new patent application, filed by Iowa University, describes a double-stranded mRNA encoding a therapeutic or prophylactic gene product. According to the inventors, this surprising design is “a form of metabolically stabilized mRNA which is approximately 1000-fold more stable than single-stranded mRNA when challenged by RNase digestion” (WO2023/014974). The distribution regarding mRNA design of new patent applications is illustrated in Figure 2.
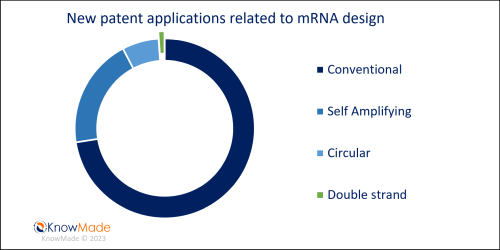
Figure 2: New patent applications in Q1 2023 – mRNA design segmentation
Newly published patent families (WO, EP, US, JP or KR) were manually segmented according to their technological features. A patent family can belong to multiple segments.
Strategy for mRNA delivery is still dominated by lipid-based carriers, and more particularly by lipid nanoparticles (LNPs) as described in the past quarter (see here). Nevertheless, the interest in polymeric carriers or extracellular vesicles as alternatives to lipid-based delivery is confirmed in the beginning of 2023, with 15% of the delivery-related new patent applications describing such carriers. The distribution regarding mRNA delivery of new patent applications is illustrated in Figure 3.
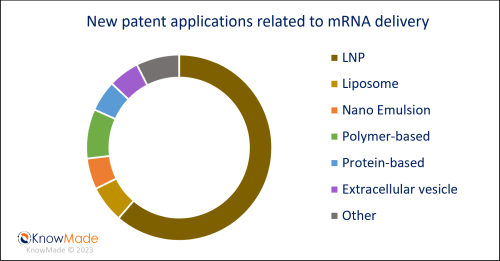
Figure 3: New patent applications in Q1 2023 – mRNA delivery segmentation
Newly published patent families (WO, EP, US, JP or KR) were manually segmented according to their technological features. A patent family can belong to multiple segments.
Q1 2023 confirms the dynamic activity in therapeutic mRNA area
With an increase of 30% in patent-filling activity, this quarter confirms that therapeutic mRNA innovation is still in an acceleration phase since 2010, as observed in KnowMade’s previous reports on mRNA vaccine (2021), mRNA cancer therapies (2023) and Self Amplifying RNA vaccine (2023). Our analysis shows that the technology was in a maturation phase up to 2010, as evidenced by a relatively low number of patent publications in all reports as illustrated in figure 4. This maturation phase was related to the problem of rapid naked RNA degradation, which was partially resolved by the incorporation of modified nucleosides and the use of lipid-based particles.
The monitoring of mRNA therapeutics allows up-to-date data on patent activity, with a quarter-tendency regarding technological segments, but also on therapeutic tendencies and novelty as information on main IP players (established or newcomers). Q1 2023 also comprises the technological analysis of key innovations such as double-stranded mRNA therapeutics, new extracellular vesicles derived from algae, or strategies to target mRNA delivery thanks to mRNA modifications developed by small companies.
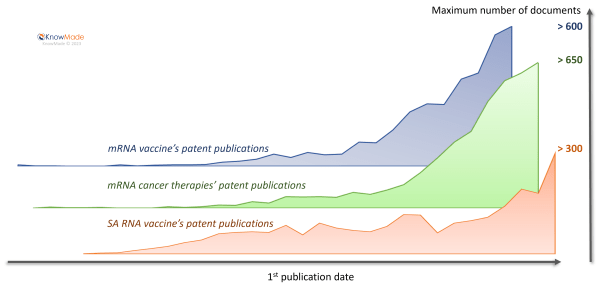
Figure 4: Time evolution of patent publications related to mRNA from KnowMade’s reports
If you need more information, reach us at contact@knowmade.fr or with our contact forms.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About the author
Elodie Bovier, PhD., works at KnowMade as a Patent Analyst in the field of Biotechnology and Life Sciences. She holds a PhD in genetic and molecular biology from the Paris Sud University. She also holds the Industrial Property International Studies Diploma (in Patent and Trademark & Design Law) from the CEIPI (Strasbourg, France).
About KnowMade
KnowMade is a technology intelligence and IP strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patent information and scientific literature into actionable insights, providing high added value reports for decision makers working in R&D, innovation strategy, intellectual property, and marketing. Our experts provide prior art search, patent landscape analysis, freedom-to-operate analysis, IP due diligence, and monitoring services.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Sensors, Semiconductor Packaging, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
