SOPHIA ANTIPOLIS, France – April 18, 2024 │ The quarterly report for the Q1 2024 Therapeutic mRNA patent monitor is now available! This report covers all aspects of mRNA design, delivery, manufacturing, storing, mRNA-based vaccines, and mRNA-based therapeutics. What’s new this quarter?
Q1 2024 IP Activity overview
The IP activity during Q1 2024 follow 2023’s trends as detailed in “A look back at mRNA patenting activity for 2023” with most significant IP activity coming from leaders in the field, such as MODERNA and BIONTECH. As in Q4 2023, see our previous press release, SANOFI belongs to the main players regarding the IP activity, especially thanks to new granted patents originally filed by TRANSLATE BIO. Additionally, 5 patents owned by CUREVAC were revoked due to non-payment of fees, representing half of the newly abandoned or expired patents.
IP activity during Q1 2024:
- 153 new patent applications published, 11 filed by MODERNA, 10 filed by BIONTECH, 4 filed by INNORNA, a Chinese company founded in 2019, specialized in mRNA delivery system, and 4 filed by ABOGEN, a Chinese company that develops cationic lipids identified as a leader in the LNP segment since the Q2 2023, see our previous press release about LNP;
- 51 newly granted patents (US/EP/JP/KR), 12 owned by MODERNA, 7 owned by SANOFI (5 originally filed by TRANSLATE BIO), and 6 owned by BIONTECH;
- 10 abandoned or expired patents, mainly abandoned CUREVAC’s patents.
This IP activity covers mRNA design, delivery, manufacturing, storing, mRNA-based vaccines, and mRNA-based therapeutics. All of these segments are analyzed within the IP monitoring activity.

Figure 1: Q1 2024 IP Activity overview
Leaders for each legal status are highlighted. ©KnowMade.
Q2 2024 ARBUTUS vs. MODERNA litigation update
The beginning of Q2 2024 is marked with ARBUTUS gain in patent ruling against MODERNA over mRNA COVID-19 vaccine. The U.S. District Court for the District of Delaware has agreed with ARBUTUS and its licensee GENEVANT on key aspects of the patent claims in question. The court’s decision supports ARBUTUS’s interpretation of the disputed patent terms related to LNP delivery system. In 2022, ARBUTUS and GENEVANT sued Moderna, alleging that it had used its LNP delivery system in its COVID-19 vaccine. A copy of the complaint, which includes a request for a jury trial, can be found here. In addition, the full details of the court’s claim construction ruling are available on the Arbutus website. Following the favorable court ruling in its patent infringement lawsuit, shares of MODERNA (MRNA.O) fell 4%, while ROIVANT’s (ROIV.O) shares gained 4% and ARBUTUS rose more than 17% on April 3.
The key aspect of ARBUTUS’s LNP technology was already highlighted in KNOWMADE’S report in 2021, see our RNA vaccine patent landscape and its extract in the figure below. Because of their importance within a dynamic technological field such as therapeutics mRNA, litigations are detailed in KNOWMADE’s monitoring activity and in some Insight such as ACUITAS vs. CUREVAC (November 2023) or ALNYLAM vs. MODERNA & PFIZER (June 2023).
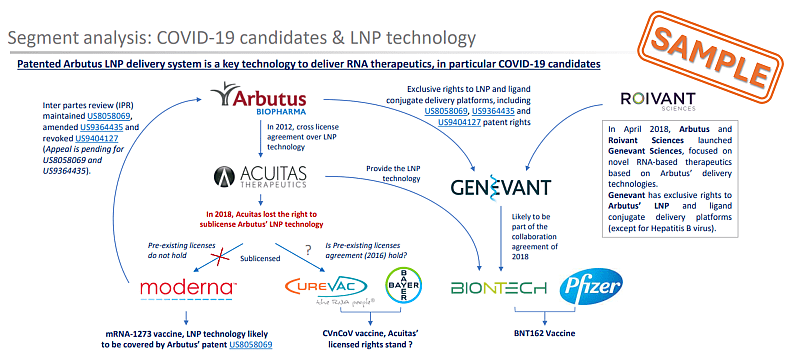
Figure 2: Sample from mRNA vaccine patent Landscape 2021 illustrating IP relationship between LNP delivery system used in COVID-19 vaccine candidates ©KnowMade.
The patent monitoring activity also allows a monitoring on therapeutic tendencies as information on main IP players (established or newcomers).
If you require additional information, please reach us at contact@knowmade.fr or with our contact form.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About the author
Elodie Bovier, PhD., works at KnowMade as a Patent Analyst in the field of Biotechnology and Life Sciences. She holds a PhD in genetic and molecular biology from the Paris Sud University. She also holds the Industrial Property International Studies Diploma (in Patent and Trademark & Design Law) from the CEIPI (Strasbourg, France).
About KnowMade
KnowMade is a technology intelligence and IP strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patent information and scientific literature into actionable insights, providing high added value reports for decision makers working in R&D, innovation strategy, intellectual property, and marketing. Our experts provide prior art search, patent landscape analysis, freedom-to-operate analysis, IP due diligence, and monitoring services.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Sensors, Semiconductor Packaging, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
