SOPHIA ANTIPOLIS, France – October 10, 2023 │ In the pharmaceutical industry, patents are crucial for companies developing new therapies and conducting clinical trials to assess their effectiveness. Given the time-consuming and expensive process of developing a new drug, patents provide legal protection to the company, guaranteeing a period of exclusivity to sell the medication and recoup their investments. Consequently, in the IP analyses conducted by Knowmade in the realm of competitive intelligence, linking a company’s clinical trials to its patents provides several insights:
- Innovation Protection: Patents allow a company to protect their discoveries and innovations. This is particularly important in the pharmaceutical industry, where clinical trials can confirm the safety and efficacy of new drugs or treatments and forecast their clinical value. If these discoveries are patented, the company can prevent others from using them without their permission. The scope of a patent protection, that depends on the prosecution (or granting) process, should cover the clinical trial application to be sure of the patent owner’s monopole position in the market. For this reason, analyzing the patent granting process, in particular the scope of the claims that give the extent of protection, in relation to clinical trials, is therefore a crucial point.
- Technology understanding: In the context of competitive technological monitoring, it is also interesting to make a connection between clinical trials and patents to have a better understanding of the technologies developed by the company. Indeed, it is possible to identify, in the patent descriptions, particularly in the examples, tests or results that have not been published in other documents, such as scientific publications related to clinical trial results.
- Development strategy: Analyzing the link between clinical trials and patents can help understand a company’s strategy and anticipate its future developments (therapeutic targets and indications considered, carriers used, etc.). This allows for identification of areas where the company has a competitive advantage, or areas where it may be vulnerable to competition.
- Financial Valuation: Investors are more inclined to invest in companies that have a patent portfolio related to the products developed by the company. This demonstrates that the company has a strong innovation strategy and is likely to generate revenue in the future. Furthermore, in the case of partnerships, M&A, having patents related to ongoing clinical trials can be a compelling argument in negotiations.
For these reasons, in Knowmade’s recent patent landscapes focused on highly innovative domain, we have conducted this kind of analysis or the main identified companies. See last Knowmade patent landscape: Allogeneic CAR patent landscape (2023), Self-amplifying RNA vaccines patent landscape (2023), mRNA cancer therapies patent landscape (2022) or Circulating DNA/RNA Patent Landscape (2021).
Fate Therapeutics’ patent families related to Allogeneic CAR clinical trials
This analysis is conducted through a comparative analysis of a company’s clinical trials (molecular target, condition, drug used, carrier, etc.) and its patent portfolio. For example, Fate therapeutic, a company founded in 2007 specialized in iPSC-derived NK cell and T-cell product candidates, was identified in the Allogeneic CAR patent landscape as a strong IP challenger. The connection between its patent portfolio and recent clinical trials was searched and is illustrated in the table.
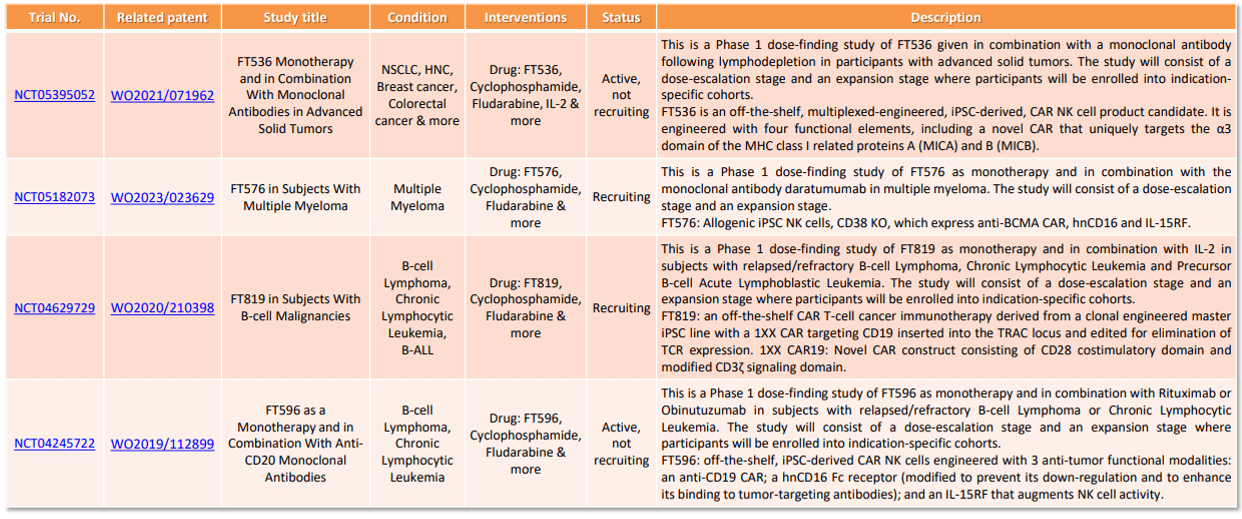
Fate Therapeutics: patent families related to clinical trials, from Allogeneic CAR patent landscape (2023)
It is interesting to note that for Fate Therapeutics, its patent families related to clinical trials are recent, filed between 2018 and 2022. Currently, in the patent applications of these families related to clinical trials, no patents have been granted in Europe or the US. The procedures are ongoing, and several amendments to the independent claims have already been made during the European process following the publication of European search opinions indicating a lack of inventive activity. The monitoring of scopes of the claims are thus strategic points to follow for Fate Therapeutics’ competitors or partners.
In conclusion, it is vital for Fate Therapeutics to obtain grants for these patents to have the broadest possible protection for these therapeutics currently in development. Indeed, in addition to the results of clinical trials which are paramount, their protection through patents is a necessary step for the development of the company.
If you need more information, reach us at contact@knowmade.fr or with our contact forms.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Sophia Antipolis, France
www.knowmade.com
About the author
Brice Sagot – CTO and co-founder of Knowmade, Brice leads the Biotechnology and Life Sciences department. He holds a PhD in Molecular Biology from the University of Nice SophiaAntipolis (France).
About KnowMade
KnowMade is a Technology Intelligence and IP Strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand their competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patent information and scientific literature into actionable insights, providing high added value reports for decision makers working in R&D, innovation strategy, intellectual property, and marketing. Our experts provide prior art search, patent landscape analysis, freedom-to-operate analysis, IP due diligence, and monitoring services.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Sensors, Semiconductor Packaging, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
