SOPHIA ANTIPOLIS, France – January 13, 2023 │ OriCiro’s synthetic biology and enzyme technologies will support Moderna’s expanding portfolio of mRNA therapeutics and vaccines. Moderna press release here.
Moderna is a major and active company in the field of therapeutic mRNA
Active development of mRNA technologies for vaccines and therapies
Messenger RNAs are under active development for various applications such as vaccines, cancer therapeutics, gene replacement, etc. KnowMade is following this technology with dedicated patent landscape analysis focused on vaccine application and cancer therapeutics and with recent insights on technology developments such as circular RNA or self-replicating RNA with patenting activity overview or Arcturus therapeutics’ platform.
Moderna is identified as a major player in both patent landscape reports with a strong portfolio comprising patent families related to numerous aspects of therapeutic mRNAs (structure, encapsulation, therapeutic applications, etc.) in addition to numerous ongoing clinical trials. Moderna is committed to not enforcing any of its patents related to the fight against COVID-19 until the end of the pandemic. However, for the past few months, Moderna has been involved in the first patent infringement battles, as recently detailed in KnowMade’s article on the Moderna vs. Pfizer BioNTech battle. In the vaccine & therapeutic mRNA ecosystem, Moderna is a key player to follow.
Moderna asset on therapeutic mRNA production
Moderna owns a broad intellectual property portfolio (over 280 patent families) and an integrated manufacturing plant that allows rapid clinical and commercial production at scale. The general process for therapeutic mRNA production includes identifying and designing the mRNA of interest, the in vitro transcription (IVT) process to obtain mRNA, encapsulating the mRNA into a carrier such as a lipid nanoparticle (LNP), and formulating the pharmaceutical composition. This process can be split into three blocks, as illustrated in Figure 1: DNA template synthesis and amplification, from which the mRNA will be transcribed in vitro; mRNA synthesis (IVT), comprising the modification for mRNA stabilization (capping, the addition of modified nucleotides, etc.); and the encapsulation/formulation process into a carrier, such as LNP. Moderna’s portfolio includes numerous patent families related to the two last steps of this whole process. Selected recent examples of such patent families are:
- The IVT process: RNA polymerase (WO2020/172239), mRNA capping (WO2022/212442), the mRNA purification step (WO2022/109171) and the industrialization of such process (WO2020/185811), for example; and
- The encapsulation process: novel lipid compounds (WO2022/204288), LNP (WO2022/204380), LNP formulation (WO2022/226318) or LNP lyophilization method, (WO2022/232585) for example.
Nevertheless, before the IVT step, a key building block is the production of the DNA matrix necessary for the IVT process. This step is less represented in Moderna’s portfolio, with only one patent family related to a bacteria strain for DNA production (WO2021/247817). The positioning of the main Modernas IP topics is illustrated in Figure 1 below.
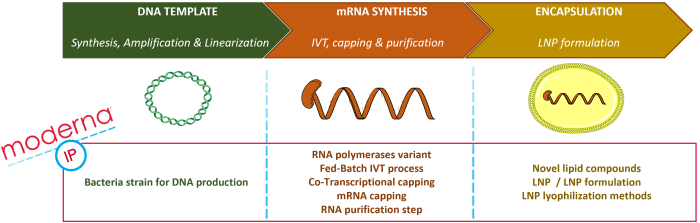
Figure 1: Moderna’s IP topics on the main steps for therapeutic mRNA synthesis.
The production of therapeutic mRNAs goes through a DNA template synthesis and amplification, the IVT (in vitro transcription) process and encapsulation/formulation. Moderna’s patent portfolio comprises numerous patent families related to the last two steps but only one related to DNA template production.
OriCiro’s portfolio could complement Moderna’s manufacturing
OriCiro Genomics, a pioneer in DNA cell-free synthesis and amplification
OriCiro Genomics, founded in 2018, is a Japanese company in Tokyo. The company formation was led by the University of Tokyo Edge Capital Partners (UTEC). OriCiro is focused on developing and commercializing cell-free synthesis and amplifying plasmid/circular DNAs for applications in gene/cell-based therapies and synthetic biology. OriCiro’s technology was invented by Dr. Masayuki Suetsugu of Rikkyo University, co-founder of the company, along his research under a program funded by the Japanese government.
OriCiro’s expertise in synthesizing and amplifying plasmid DNA, a key building block in mRNA manufacturing, could complement Moderna’s manufacturing expertise and further accelerate its future R&D activity. Indeed, OriCiro’s technology advantage is mainly its cell-free production process, avoiding numerous steps necessary in a cell-based DNA amplification process. Moreover, the classical PCR DNA amplification process is not easily applicable to circular DNA amplification. Indeed, it needs specific primers for each circular DNA to amplify and results in linear DNA that will need to be circularized for IVT. OriCiro’s technology, therefore, provides a cell-free cloning tool that overcomes cell-based and PCR process constraints, and it could be a valuable platform for creating large DNA constructs.
OriCiro’s patent portfolio overview
OriCiro’s patent portfolio comprises six patent families filed between 2015 and 2020. These patent families consist of 63 documents divided into granted patents (41%) and pending patent applications (59%). OriCiro actively collaborates with Japanese academics. Indeed, four patent families are co-owned by the Japan Science and Technology Agency (JSTA), and one is co-owned by the University of Tokyo. The geographical coverage of this portfolio, as illustrated in Figure 2, is mainly focused on the triadic territories (US, EP, JP).
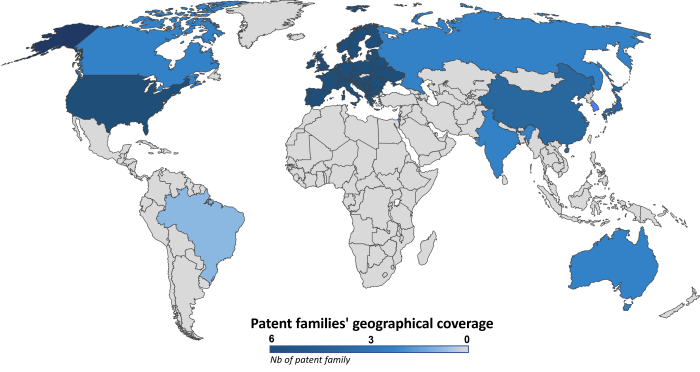
Figure 2: Geographical coverage of patent families related to cell-free amplifying circular DNA.
OriCiro’s IP on cell-free amplifying circular DNA
In its patent portfolio, OriCiro holds three patent families related to a method of cell-free amplifying circular DNA, filed between 2015 and 2018 in collaboration with the JSTA and Dr. Masayuki Suetsugu, named as the inventor. These three patent families cover the territory of interest for Moderna since they cover the United States, the European area, and Japan, among other territories. The main legal information on these areas, with links to the PDF files, is summarized in the table below.
| Topics | Family numbers | First application dates | Granted | Pendings |
| General principles | WO2016/080424 | 2015-11-18 | EP3222717 US10301672 JP6262877 |
|
| Temperature improvements | WO2017/199991 | 2017-05-17 | JP6764193 | US20190276883 EP3460058 |
| Separation step improvements | WO2018/159669 | 2018-02-28 | JP6960684 US7911445 |
EP3591051 |
Table 1: OriCiro’s patent families related to circular DNA cell-free amplifying.
The first patent family relates to the general principle of the enzymatic process of amplifying circular DNA (WO2016/080424). The invention is an enzymatic method for amplifying circular DNA in a single reaction mixture comprising a circular DNA with a replication origin sequence (oriC) as a template and three groups of enzymes. These groups of enzymes are (1) enzymes that catalyze the replication of circular DNA, (2) enzymes that catalyze Okazaki fragment maturation and synthesize two sister circular DNAs constituting a catenane, and (3) enzymes that catalyze the separation of the two sister circular DNAs.
The second patent family is an improvement of the initial invention focused on the condition of temperature (WO2017/199991). Indeed, the initial process is completed by a temperature cycle of repeating incubation to promote efficient circular DNA amplification. This cycle is a first step at 30 to 80°C to initiate the replication of circular DNA comprising oriC, and an incubation step at 10 to 27°C, to suppress replication initiation while the DNA elongation reaction progresses. The incubation time will depend on the circular DNA’s size, and the number of temperature cycles is not limited.
Finally, the third patent family, WO2018/159669, is also an improvement of the initial invention with a focus on the step that separates the two sister circular DNAs. This step is improved with a reduction of DNA multimers by two nonexclusive technical solutions:
- Utilizing a replication termination mechanism using the system ter-Tus. In this solution, the circular DNA matrix should include a pair of ter sequences, each inserted outward with respect to oriC; and
- Utilizing a DNA multimer separation mechanism with a site-specific recombination system, such as dif-XerCD. In this solution, the circular DNA matrix should include a nucleotide sequence recognized by a DNA multimer separation enzyme (in any position on circular DNA).
The model of OriCiro’s method, according to these patent families, is illustrated in Figure 3 below. In addition, characteristic elements of each patent family are also marked.
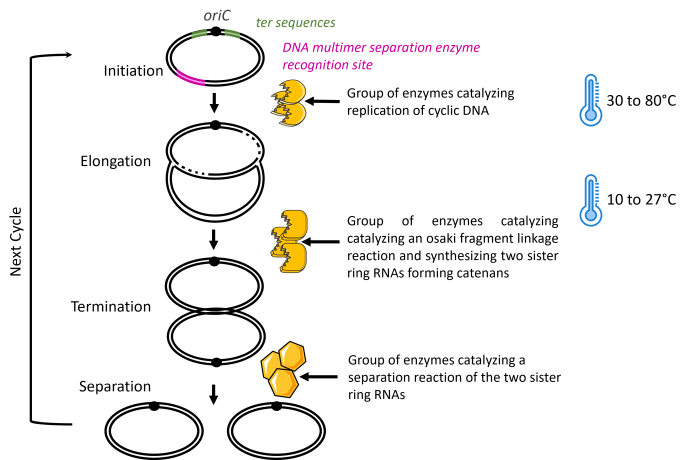
Figure 3: Model of the cell-free amplifying circular DNA in OriCiro’s Invention.
The three groups of enzymes described in the patent family WO2016/080424 are illustrated in yellow. The temperature specifications from the patent family WO2017/199991 are illustrated in blue. Finally, the supplemental sequences needed for the separation step, an improvement described in the patent family WO2018/159669, are illustrated in green and pink.
OriCiro’s most recent patent families
In addition to the previously described patent families, OriCiro holds three other patent families related to DNA synthesis/amplification. It is worth noting that the most recent patent family filed in collaboration with the University of Tokyo is the first related to a medical application. It describes the diagnosis of neuromuscular disease through amplification, after circularization, of a DNA fragment from a subject (WO2022/018881). OriCiro’s patent portfolio is completed by a patent family related to a method for editing DNA in a cell-free system (based on a circular DNA amplification process) (WO2020/027110) and a DNA fragment joining method and kit (WO2019/009361).
OriCiro’s acquisition will reinforce Moderna’s mRNA platform
With OriCiro’s acquisition, Moderna will reinforce its mRNAs platform. Indeed, OriCiro’s patent portfolio comprises intellectual property rights, know-how, and expertise complementary to that of Moderna because it brings an additional block upstream to the production steps already developed by Moderna, i.e., the production of the DNA matrix necessary for the IVT process.
Moreover, OriCiro’s IP rights seem relatively strong. Indeed, the patent family covering the general principle of OriCiro’s technology (WO2016/080424) has been granted in the US, EP, and JP with a broad set of claims and close to the set of claims in the application as filed. Moreover, the two other patent families related to DNA matrix production are improvement patent families (WO2017/199991 and WO2018/159669). Despite the fact that these patent families are still pending, according to international search reports and preliminary reports on patentability, claimed invention seems to be novel, to involve an inventive step, which is encouraging for patent obtention.
Explore KnowMade’s IP expertise in medical sciences: healthcare patent landscape.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About the author
Elodie Bovier works at KnowMade as a Patent Analyst in the field of Biotechnology and Life Sciences. She holds a PhD in genetic and molecular biology from Paris Sud University. She also holds an Industrial Property International Studies Diploma (in Patent and Trademark & Design Law) from the CEIPI (Strasbourg, France).
About Knowmade
KnowMade is a Technology Intelligence and IP Strategy consulting company specialized in analysis of patents and scientific information. The company helps innovative companies and R&D organizations to understand their competitive landscape, follow technology trends, and find out opportunities and threats in terms of technology and patents.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patents and scientific information into business-oriented report for decision makers working in R&D, Innovation Strategy, Intellectual Property, and Marketing. Our experts provide prior art search, patent landscape analysis, scientific literature analysis, patent valuation, IP due diligence and freedom-to-operate analysis. In parallel the company proposes litigation/licensing support, technology scouting and IP/technology watch service.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Solid-State Sensors/Actuators, Semiconductor Manufacturing, Packaging & Assembly, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
