SOPHIA ANTIPOLIS, France – August 09, 2022 | KnowMade noticed recent patenting activity in the field of circular mRNA therapeutics, inspiring us to publish a brief statement about this therapeutic technology.
What is a circular mRNA?
CircRNA structure & function
The first known circular RNA (CircRNA) function is a regulatory function (through miRNA regulation, transcription regulation, gene expression regulation, immune system regulation). Recently, a coding function was found in some circular RNAs. These CircRNAs comprise internal ribosome entry sites (IRESs) which launch the cap-independent translation and an open reading frame (ORF) encoding a protein as illustrated in the figure below. Functions associated with CircRNA encoded proteins are still under investigation – some were associated with synapse and muscle functions, and also with cancer development.
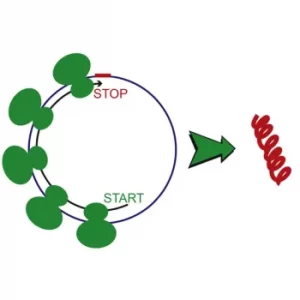
Figure 1: Illustration from Pamudurti et al., Translation of circular RNA, Molecular Cell 66, 9–2, 2017.
CircRNA advantage in therapeutic approaches
Circular structures are of interest in developing new mRNA-based therapeutic approaches. Indeed, because of their circular structure, CircRNAs are resistant to RNase action, resulting in strong stability, and are also characterized by a potential immunogenicity that can be a drawback or an advantage depending on the therapeutic approach (i.e., it might be useful in vaccination strategy but not for protein replacement strategy).
Few therapeutic circular mRNA companies, for now
Circular mRNA for therapeutic uses is an emerging field in the recently developed mRNA therapeutics approach. For this reason, companies currently developing therapeutic circular mRNA are few and very recent. Here we have listed the current most relevant industrial players in this field.
THERORNA is developing a circular RNA vaccine against SARS-CoV-2 and emerging variants
THERORNA, a Chinese company founded in Beijing in April 2021, completed a US $42 million Series A financing round in June 2022. The company focuses on leveraging its proprietary CircRNA technology platform to develop next-generation vaccines and innovative therapies, particularly a vaccine against SARS-CoV-2.
The scientific founder of THERORNA, Professor Wensheng Wei, is named as inventor in a patent family, WO2022/037692, filed by Beijin University on August 20, 2021. The opinion of the International Searching Authority is positive for a circular RNA comprising a nucleic acid sequence encoding an antigenic polypeptide wherein said polypeptide comprises a receptor-binding domain (RBD) of the S protein.
A detailed analysis of the experimental part of the patent application shows that the circular RNA of the invention has resistance to nuclease, stability for two weeks at room temperature in an LNP formulation and when transfected to human and mouse cells, and demonstrates the ability to express a functional SARS-CoV-2 RBD of an S protein. Moreover, it induces a SARS-CoV-2 specific immune response in mice and generates a high level of specific neutralizing antibodies that are effective against the B.1.351 virus strain in an authentic virus challenge at seven weeks post-boost dose. These results are also listed and supplemented with data on rhesus macaques in the recently published scientific publication (May 2022): “Circular RNA vaccines against SARS-CoV-2 and emerging variants”, Liang Qu et al., Cell, Volume 185, Issue 10.
This CircRNA is one of the first CircRNA for COVID-19 vaccination.
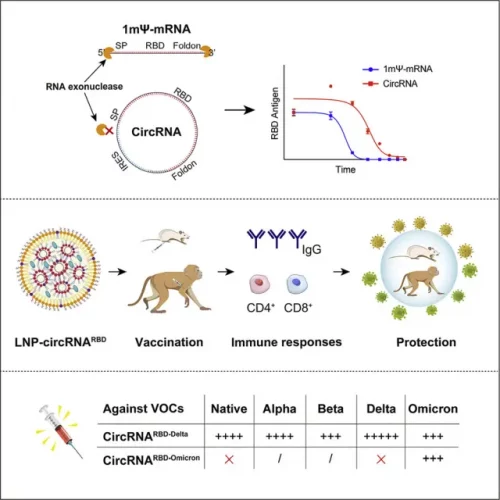
Figure 2: Illustration from Liang Qu et al., 2022.
LARONDE holds the largest patent portfolio
In May 2021, Flagship Pioneering launched LARONDE, a platform company developing Endless RNA™ (eRNA), a circular engineered form of RNA that can be programmed to express therapeutic proteins. Flagship initially committed $50 million to support the development of LARONDE’s platform and an initial pipeline of new medicines, and in August 2021, LARONDE raised $440 million in Series B financing.
LARONDE’s IP rights are owned by Flagship Pioneering, and this portfolio consists of a patent pool covering aspects from CircRNA structure, a delivery road to applications such as vaccination, heterologous protein expression, and therapeutic uses, within 12 patent families filed since December 2018.
This portfolio comprises three granted patents in the US (US11160822, US11058706, and US10953033) belonging to the same family and covering a method for expressing in a subject a functional polypeptide encoded in a circular RNA for at least a seven-day period, a method for making a pharmaceutical composition comprising a circular RNA, and a method for providing a subject with circular RNA, respectively. This family comprises an international patent application (WO2019/118919) claiming a therapeutic circular RNA.
Most recent patent families relate to SARS-CoV-2 therapeutics such as CircRNA for vaccination, for monoclonal antibody production in humanized mammals (bovine & caprine) with experimental data on non-human mammals and on humans (WO2022/051629, WO2021/236980, WO2021/236952, and WO2021/236930), a delivery road such as topical, nasal, intramuscular & intravenous (WO2022/051629), or expression of multiple immunogens in a subject by administering multiple circular RNA molecules (WO2021/236930).
The rest of the patent portfolio relates to CircRNA structure (WO2021/155175), a delivery system (WO2021/155171), a circRNA dosing method for maintaining the encoded protein expression (WO2020/257727), Chimeric Antigen Receptors (CAR) therapy (WO2020/252436), modification to decrease CircRNA immunogenicity (WO2020/198403), CircRNA preparation (WO2020/181013), and CircRNA for cosmetic uses (WO2020/180752).
ORNA therapeutics leads CircRNA-based Chimeric Antigen Receptors (CAR) therapy
In early 2021, ORNA Therapeutics, founded by MIT researchers, launched with over $100 million raised to develop a new class of fully engineered circular RNA therapies, named oRNAs, to treat a wide range of diseases including cancer, autoimmune diseases, and genetic disorders.
ORNA’s leading program is an in-situ Chimeric Antigen Receptors (CAR) therapy that combines oRNAs and custom engineered LNPs to create modified immune cells within the patient for treating cancer (isCARTM corresponding to an anti-CD19 CAR). The company has also developed a proprietary FoRCE screening platform, allowing ORNA to discover many novel IRES elements that drive oRNA expression to levels well above those of standard IRES elements, including some that show differential activity across cell types. In addition, ORNA has extended its oRNA-LNP technology into several other indications including Duchenne Muscular Dystrophy (delivering full-length gene therapy to patients) and vaccines (intramuscularly administered immunotropic LNPs for vaccine applications, including for COVID-19).
The ORNA patent portfolio comprises six patent families related to circular mRNA compositions and methods, all still under examination and filed since May 2020. Five are international patent applications (WO2020/237227, WO2021/113777, WO2021/189059, WO2021/226597 and WO2021/236855) and two are US patent applications (US20220177540 and US20210371494). All of these patent applications disclose experimental data on CircRNA structure (IRES elements, spacers, sequence modifications, stability), CircRNA-LNP formulations and delivery efficiency for CAR therapy to create modified immune cells for treating cancer. Another experimentally tested therapeutic is the production of a CircRNA encoding dystrophin (WO2021/189059).
CircRNA, still an emerging field
The emerging field of circular mRNA appears to be still full of blank spaces, with companies that seem to specialize in each specific therapeutic approach. The Chinese company THERORNA appears to have the lead on circular RNA vaccines against SARS-CoV-2 and emerging variants, with convincing experimental results and covered by a promising international patent application. ORNA appears to be the best positioned on circular RNA for CAR therapy against cancer, with a strong portfolio on LNP-based CircRNA formulations for efficient delivery. Finally, LARONDE owns the largest and oldest IP portfolio covering a wide range of therapeutic applications, putting this company in an apparent leading position.
Nevertheless, other mRNA companies like MODERNA, CUREVAC or TRANSLATE BIO have mentioned CircRNA as an alternative to their therapeutic linear mRNA inventions, but with no experimental data, revealing a more protective approach by patent drafting rather than a technological expertise.
Find all KnowMade patent landscape reports on healthcare.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About the author
Elodie Bovier works at Knowmade as a Patent Analyst in the field of Biotechnology and Life Sciences. She holds a PhD in genetic and molecular biology from Paris Sud University. She also holds an Industrial Property International Studies Diploma (in Patent and Trademark & Design Law) from the CEIPI (Strasbourg, France).
About Knowmade
Knowmade is a Technology Intelligence and IP Strategy consulting company specialized in analysis of patents and scientific information. The company helps innovative companies and R&D organizations to understand their competitive landscape, follow technology trends, and find out opportunities and threats in terms of technology and patents.
Knowmade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patents and scientific information into business-oriented report for decision makers working in R&D, Innovation Strategy, Intellectual Property, and Marketing. Our experts provide prior art search, patent landscape analysis, scientific literature analysis, patent valuation, IP due diligence and freedom-to-operate analysis. In parallel the company proposes litigation/licensing support, technology scouting and IP/technology watch service.
Knowmade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Solid-State Sensors/Actuators, Semiconductor Manufacturing, Packaging & Assembly, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
