SOPHIA ANTIPOLIS, France, January 4, 2023 │ An alternative synthetic mRNA platform — self-amplifying RNA (saRNA)— for vaccines may allow smaller doses of vaccines to be administered. Well positioned on this technology, Arcturus Therapeutics, a San Diego-based company founded in 2013, has a self-amplifying RNA vaccine pipeline for SARS-CoV-2 (COVID-19) and influenza. In December 2022, CSL Seqirus, an Australian biotech company focused on influenza vaccines, announced the closing of its collaboration and licensing agreement with Arcturus Therapeutics. The agreement grants access to the Arcturus Therapeutics saRNA vaccine platform technology, which recently reported results from a large COVID-19 phase 3 vaccine efficacy study, meeting its primary and secondary endpoints on the prevention of infection and severe disease with a favorable safety and tolerability profile. Under the terms of this agreement, Arcturus Therapeutics will receive an upfront payment of US$200 million and will be eligible to receive further payments dependent upon the achievement of certain development and commercial milestones along with royalties and profit sharing on future product sales. Accordingly, the intellectual property of Arcturus Therapeutics on saRNA technology is important to better understand the technology developed by the company. Arcturus Therapeutics’ patent portfolio comprises 46 patent families, and the company owns four patent families (WO2020191103, WO2022056413, WO2022036170, WO2021183563), published between 2020 and 2022, describing inventions closely related to its saRNA vaccine platform.
Method for lipid-encapsulating large saRNA
The patent application WO2020191103 (pending in the US, EU, JP, KR, IN, AU, and others) filed in 2020 describes a method for making lipid-encapsulated RNA. Although this patent family is not focused on self-amplifying RNA technology, examples 5 and 6 describe methods for producing lipid-encapsulated RNA nanoparticles containing long-length mRNA (a characteristic of saRNA). The results in the Table below show that mRNA of about 800 to 12,000 nucleotides can be packaged with about 95–97% encapsulation using the process described in this patent application.
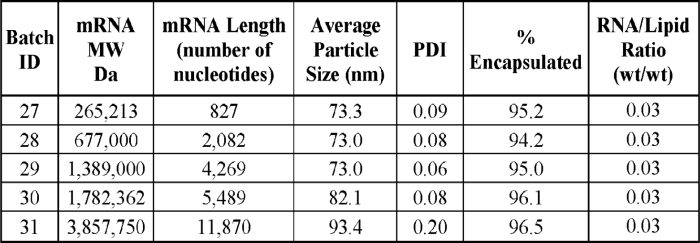
Figure 1: Encapsulation rate as a function of mRNA size (WO2020191103).
Using this method, lipid compositions were formulated in the range cationic lipid:DSPC:Cholesterol:PEG-DMG, 48-60:5-10:28-38:0.5-3.0 mol%. The lipid solution was pumped from the reservoir through a mixing module by an HPLC pump through tubing with a 0.01” lipid inlet at flow rates from 25 to 87.5 mL/min. The RNA solution was pumped from the reservoir through an HPLC pump module through tubing with a 0.132” RNA inlet. The flow rate of the RNA was three times the flow rate of the lipids, and the output of the mixing was a 25% EtOH solution. The output solution was diluted in buffer, and free RNA and ethanol were removed by tangential flow filtration. The figures below schematically show the overall process outlining the production steps.
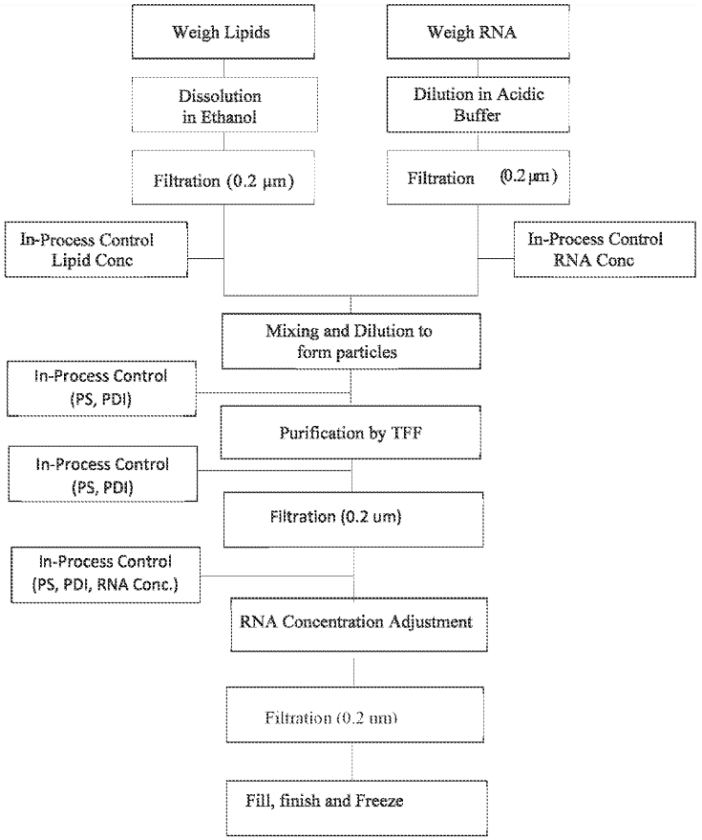
Figure 2: Flow chart for one embodiment of a process of producing lipid nanoparticles (WO2020191103).
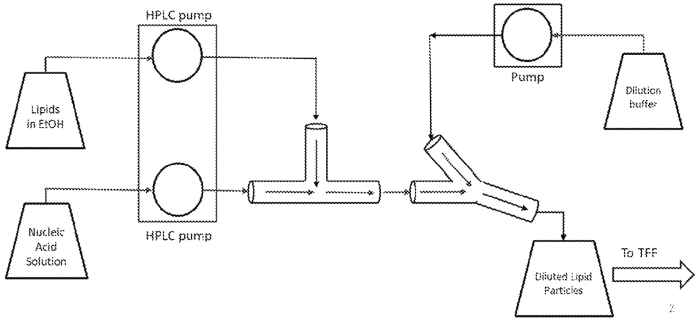
Figure 3: Apparatus for producing lipid-encapsulated RNA nanoparticles (WO2020191103).
The patent application WO2022056413 (pending in the US and EU at the moment) filed in 2021 is an improvement of the process described in the previous patent application WO2020191103 for encapsulating larger mRNAs. Indeed, methods using LNPs with larger RNAs (more than 6,000 nucleotides) may have poor size, dispersion, and RNA degradation due to shear forces. Therefore, in this recent invention, the lipid-encapsulated RNA nanoparticles’ well-defined shape and reproducible size are prepared using an aqueous RNA solution flow rate that reduces shear forces and allows the integrity of the large RNA to be preserved. To do so, the addition rate for each solution is controlled using a high-pressure piston pump, with the lipid and mRNA solutions being added at a flow rate of 30-75 and 90-225 mL/min, respectively. The two streams converge in a stainless-steel mixing module at a total flow rate of 120-300 mL/min. In an example, inventors show that a lower flow rate worked better for large RNA-encapsulated formulations for both the multi-inlet vortex mixer system (MIVM) and the Arcturus Therapeutics (ARC) formulation system. In this experiment, a composition of Ionizable Cationic Lipid: DSPC: CHOL: PEG-DMG2000=50:10:38.5:1.5 molar ratio with a total lipid: RNA weight ratio of about 35.78:1 was used in this example. Luciferace saRNA (9693 nucleotides) was used. With the lower flow rate, particle size (PS) and polydispersity index (PDI) were smaller, and percentage encapsulation was higher (see Table below). To attain an acceptable manufacturing rate and acceptable LNP quality, 100 mL/min for the MIVM system and 160 mL/min for the ARC system were chosen for further development.
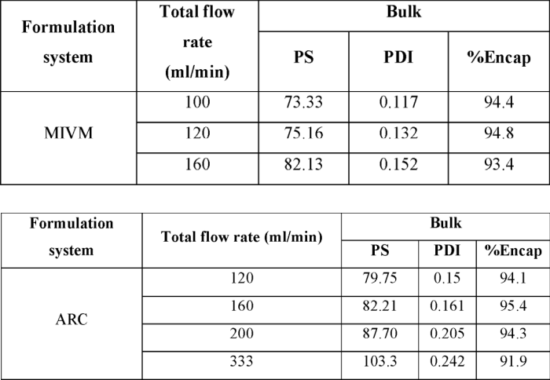
Figure 4: Effect of flow rate in MIVM and ARC systems with saRNA (WO2022056413).
Other improvements are also disclosed in this patent application. For example, a higher NaCl concentration (up to 300 mM) in citrate buffer was needed for a higher in-process concentration formulation, and it provided small particle size, PDI, and a high percentage of encapsulation. However, higher NaCl concentrations did not need to be increased once a threshold was reached. Moreover, pH 4.0 citrate buffer was chosen because the pH 4.0 buffer also maintained better mRNA purity and integrity.
Method of lyophilizing LNP-saRNA
The efficiency of the lyophilization process is a critical step for therapeutic LNP encapsulating saRNA conservation, and the patent application WO2022036170 (pending in the US and EU at the moment) describes a method of lyophilizing a composition comprising lipid nanoparticles encapsulating saRNA (11,000 nucleotides in the example). This patent application is well-detailed, and the quality of lyophilized lipid nanoparticle formulations was assessed by analyzing the formulations post-lyophilization and comparing this to the lipid nanoparticle formulation before lyophilization as well as after a conventional freeze/thaw cycle (i.e., frozen at -70°C. then allowed to thaw at room temperature).
The lipid nanoparticle formulation comprising saRNA was processed with a buffer exchange to form a pre-lyophilization suspension with a concentration of 0.05 to 2.0 mg/mL saRNA, 0.01 to 0.05 M potassium sorbate, 0.01 to 0.10% w/v Poloxamer 188 (Kolliphor®), 14 to 18% w/v sucrose, 25 to 75 mM NaCl, and 15 to 25 mM pH 8.0 Tris buffer. The pre-lyophilization formulation was then lyophilized in a Millrock Revo Freeze Dryer (Model No. RV8554), using aliquots of 2.0 mL of suspension and the lyophilization cycle provided in Table below.
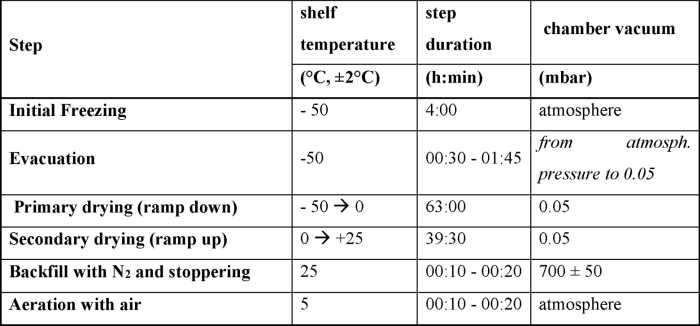
Figure 5: Lyophilization cycle for saRNA-Lipid nanoparticle formulation (WO2022036170).
The lyophilized particles prepared were reconstituted in 2 mL of water and characterized. The results in the Table below show that the lyophilized compositions were found to produce lyophilized lipid nanoparticle formulations with adequate size, polydispersity, and delta values (~5.3 nm) upon reconstitution. The percentage of saRNA encapsulation was 97% for a pre-lyophilization sample and 93% for a post-lyophilization sample.

Figure 6: SaRNA-Lipid Nanoparticle – Characteristics Pre- and Post-lyophilization (WO2022036170).
Coronavirus vaccine compositions
The patent application WO2021183563 (pending in the US, EU, and elsewhere) describes coronavirus vaccine compositions and this invention is strongly related to the ARCT-021 vaccine. Scientific results of this invention were also published in the article, “A single dose of self-transcribing and replicating RNA-based SARS-CoV-2 vaccine produces adaptive protective immunity in mice”, available here. In this article, the vaccine is named LUNAR-COV19 and ARCT-021 in the patent application. These documents show that a single prime vaccination in mice led to robust antibody responses, with neutralizing antibody titers increasing up to day 60. Findings suggest the potential of LUNAR-COV19 (ARCT-021) as a single-dose vaccine. STARR™ construct (see Figure below), and all sequences used in STARR™ SARS-CoV-2 RNA are disclosed in this patent application and give several complementary insights compared to a scientific article (see Table 8 of the patent application). Moreover, lipid nanoparticle manufacture and the lyophilization process are also described.
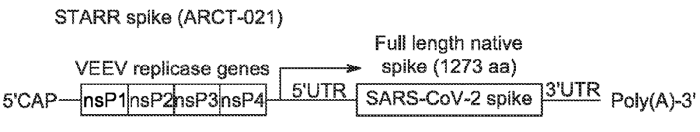
Figure 7: Schematic diagram of the SARS-CoV-2 self-replicating STARR™ RNA. The STARR™ construct encodes for the four non-structural proteins, nsl-ns4, from Venezuelan equine encephalitis virus (VEEV) and the SARS-CoV-2 full-length spike (S) protein (WO2021183563).
The claims are currently pending, relatively broad, and can encompass the coronavirus or influenza vaccine, and the procedure is ongoing. This patent family is a major element protecting the intellectual property of the Arcturus Therapeutics saRNA vaccine. It will be important for the company to obtain the broadest possible protection for this invention.
Arcturus Therapeutics could take a more significant place in the field of saRNAs
Currently, all patent families closely related to the self-amplifying RNA vaccine developed by Arcturus Therapeutics are pending. The potential grant, scope of claims obtained, and geographic coverage of these patent applications will be critical for Arcturus Therapeutics to obtain protection for its coronavirus and influenza vaccines. These patent applications currently cover saRNA encapsulation, lyophilization methods, and the use of these RNA products as vaccines for infectious, especially COVID-19 and influenza.
As observed in a previous article by KnowMade, the main players leading conventional RNA vaccines (i.e., Moderna, BioNTech, and CureVac) are weak or not present in the field of saRNA, which is led by players such as GSK, Gritstone, or Janssen Biotech. It is also interesting to observe the arrival of several startups focusing on this technology, such as VaxEquity and Replicate Bioscience. In this context, Arcturus Therapeutics is an active player in messenger RNA medicines and vaccines. It will be essential to monitor its development because it could take a more significant place in the field of saRNAs.
This article is an insight of KnowMade’s expertise, discover all our healthcare patent landscapes.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About the author
Brice Sagot, CTO and co-founder of KnowMade, leads the Biotechnology and Life Sciences department. He holds a PhD in Molecular Biology from the University of Nice SophiaAntipolis (France).
About KnowMade
KnowMade is a Technology Intelligence and IP Strategy consulting company specialized in analysis of patents and scientific information. The company helps innovative companies and R&D organizations to understand their competitive landscape, follow technology trends, and find out opportunities and threats in terms of technology and patents.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patents and scientific information into business-oriented report for decision makers working in R&D, Innovation Strategy, Intellectual Property, and Marketing. Our experts provide prior art search, patent landscape analysis, scientific literature analysis, patent valuation, IP due diligence and freedom-to-operate analysis. In parallel the company proposes litigation/licensing support, technology scouting and IP/technology watch service.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Solid-State Sensors/Actuators, Semiconductor Manufacturing, Packaging & Assembly, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
