SOPHIA ANTIPOLIS, France – April 28, 2025 │ The therapeutic RNA landscape continues to evolve with a robust pace of innovation. The latest Q1 2025 edition of our Therapeutic mRNA patent monitor highlights significant developments in intellectual property activities, shedding light on key contributors and trends in this dynamic field.
BioNTech, Sanofi, Penn, and Moderna Lead mRNA Patent Landscape in Q1 2025
The therapeutic mRNA sector continues to gain momentum, with a strong start to the year reflecting growing global interest and investment in this cutting-edge field. The first quarter of 2025 saw the publication of 257 new patent applications—a substantial increase from the 153 applications recorded in Q1 2024. This surge in activity signals a sustained upward trend in intellectual property developments within the field. BIONTECH led the way with 24 applications, including four co-filed with the UNIVERSITY OF PENNSYLVANIA, reaffirming their strategic academic partnership. Sanofi followed with 12 filings, while the University of Pennsylvania contributed 11 applications in total—including the four co-filed with BIONTECH—and MODERNA added five, see figure 1. All of these organizations remain key players in the mRNA therapeutics landscape, as identified in our 2023 and 2024 annual summaries.
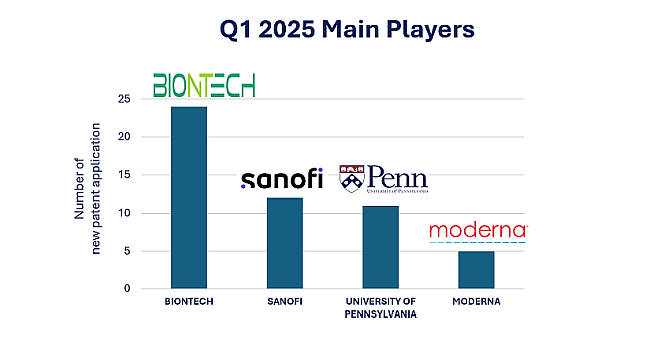
Figure 1: Q1 2025 Patent Filings by Key mRNA Therapeutics Innovators
A significant proportion of these filings—nearly half—focus on innovations in therapeutic mRNA delivery systems, underscoring the industry’s efforts to optimize efficacy and targeting. Vaccination remains the leading therapeutic application area, with BIONTECH’s filings notably addressing vaccine candidates for HIV, herpes, and malaria. These developments highlight both the breadth of ongoing research and the strategic diversification of mRNA-based approaches across major infectious diseases.
Expansion of Enforceable IP Rights Reflects Growing Maturity of mRNA Sector
In addition to the surge in published applications, the first quarter of 2025 also marked a notable increase in the number of newly granted patents in key jurisdictions (US, EP, JP and KR). A total of 64 first-time grants were recorded during the quarter, nearly doubling the 33 new inventions granted in Q1 2024. This significant rise underscores the maturation of R&D efforts in therapeutic mRNA and the progression of early-stage innovations into enforceable rights. The United States accounted for the largest share with 26 new granted patents, followed by Japan with 19, South Korea with 10, and Europe with 9. This geographic distribution reflects the sustained global interest and investment in securing intellectual property around therapeutic mRNA technologies across major innovation hubs.
Emerging Players in mRNA Therapeutics: Early Patent Filings by PINION IMMUNOTHERAPEUTICS and ARCALIS in Q1 2025
The activity of monitoring intellectual property not only enables tracking the evolution of established players but also helps identify the emergence of new entrants in the market through their first patent filings. During the first quarter of 2025 (Q1 2025), several companies making their first patent filings in this field were identified, including PINION IMMUNOTHERAPEUTICS and ARCALIS. Early identification of these new players is essential to anticipate competitive dynamics, detect new technological trends, and potentially uncover opportunities for collaboration or investment.
PINION IMMUNOTHERAPEUTICS
PINION IMMUNOTHERAPEUTICS (formerly ARV TECHNOLOGIES) is a biotechnology company based in Rockville, Maryland, founded in 2024. Specialized in the development of mRNA vaccines, it leverages artificial intelligence and structural biology to design innovative antigens, combined with advanced lipid nanoparticle (LNP) formulations. This approach aims to provide effective treatments against chronic viral infections and certain cancers. The leadership team includes renowned experts such as Dr. Gregory Glenn, CEO, who contributed to the development of the Nuvaxovid vaccine at Novavax, and Dr. Jianzhu Chen, co-founder and Professor of Immunology at MIT. Its patent application US20250092083, co-filed with the Chinese company SUZHOU VENCUBIO, aims to develop a new class of sterol-derived ionizable lipids intended for LNP formulations capable of efficiently delivering therapeutic agents, particularly nucleic acids such as mRNA, siRNA, saRNA, and plasmid DNA.
The lead compound, ARV-T1, was compared to the commercial lipid SM-102 and showed:
- Efficient mRNA encapsulation,
- Higher expression of the SARS-CoV-2 Spike protein in 293T cells,
- Increased transfection of GFP mRNA in BHK cells,
- More sustained in vivo luciferase expression in mice.
It also induced stronger humoral (total IgG, neutralizing antibodies) and cellular (antigen-specific T cells) immune responses. Moreover, ARV-T1 enabled efficient mRNA delivery in transgenic mice, targeting multiple immune cell types. It also performed well in plasmid DNA transfection, gene silencing by siRNA, and prolonged expression via saRNA. Finally, structural analogs (ARV-T11, T12, T13) were synthesized and characterized (NMR, HPLC, MS) and were found to be as effective or even superior to ARV-T1 and SM-102 in terms of transfection efficiency, encapsulation, and LNP size.
ARCALIS
ARCALIS is a Japanese company founded in 2021, specialized in the development and contract manufacturing (CDMO) of mRNA vaccines and therapies. Resulting from a joint venture between Axcelead, Inc., Japan’s leading drug discovery platform, and Arcturus Therapeutics, a U.S. biotechnology company specializing in mRNA-based medicines, ARCALIS offers integrated services ranging from mRNA design to cGMP-compliant manufacturing, including LNP formulation and analytical testing. In July 2023, the company inaugurated a manufacturing facility in Minamisoma, dedicated to the production of mRNA drug substances, with the ambition to commercialize, starting in 2024, the first approved vaccine using self-amplifying mRNA technology. In November 2024, Meiji Seika Pharma invested in ARCALIS to strengthen domestic mRNA vaccine production in Japan, notably the KOSTAIVE® COVID-19 vaccine. The patent application US20250076306, co-filed with HITACHI, aims to optimize mRNA (or DNA) sequences encoding a target protein by associating each candidate sequence (for example, varying UTRs or codons) with a unique “barcode” peptide, co-expressed with the protein of interest. After expression, the peptides are enzymatically released and analyzed by mass spectrometry, enabling indirect quantification of protein expression based on the mRNA sequence used. This allows multiplexed analysis — many sequence combinations in a single measurement.
- Example 1 validates this approach using two UTRs for eGFP, showing that peptide intensity reflects expression level.
- Example 2 demonstrates large-scale application with 9 UTR+ORF combinations and over 2 million possible barcode peptides, randomly combined via DNA Assembly, then analyzed after bacterial amplification, sequencing, and mass spectrometry.
This system thus enables high-throughput screening of optimal sequences with correction of experimental biases, for applications in biotechnology, vaccines, or gene therapy.
Why subscribe to this monitor?
Innovative and dynamic sectors like mRNA therapeutics require clear visibility into the evolving IP landscape. A patent monitoring solution offers companies a strategic advantage, delivering up-to-date and focused intelligence on emerging patents and technological trends. Our tailored services support organizations in identifying new opportunities for collaboration, reinforcing protection of their inventions, and confidently navigating IP complexities. With these insights, businesses are better positioned to shape their innovation strategies and sustain long-term growth in competitive environments. For more detailed insights into these developments and how they may impact your business, please contact us.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About KnowMade
KnowMade is a Technology Intelligence and IP Strategy consulting company specialized in analysis of patents and scientific information. The company helps innovative companies and R&D organizations to understand their competitive landscape, follow technology trends, and find out opportunities and threats in terms of technology and patents.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patents and scientific information into business-oriented report for decision makers working in R&D, Innovation Strategy, Intellectual Property, and Marketing. Our experts provide prior art search, patent landscape analysis, scientific literature analysis, patent valuation, IP due diligence and freedom-to-operate analysis. In parallel the company proposes litigation/licensing support, technology scouting and IP/technology watch service.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Solid-State Sensors/Actuators, Semiconductor Manufacturing, Packaging & Assembly, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
