SOPHIA ANTIPOLIS, France – October 10, 2022 │ Since 2020 and the COVID-19 crisis, mRNA technology has experienced a big boost in interest thanks to the outstanding results of vaccines produced by Moderna and Pfizer/BioNTech. Indeed, the threat of the COVID-19 pandemic accelerated rapid-response vaccine development. mRNA-based therapeutics were shown to deliver on their promise: active at a relatively low dose range, they can be developed rapidly, and the GMP-compliant manufacturing processes are easily upscaled for rapid availability of large numbers of doses. The success of the COVID-19 mRNA vaccine opened the door for numerous therapeutic applications.
Indeed, along with vaccines against viruses (see RNA vaccine report – 2021), mRNA appears a good candidate for cancer treatment. Today, the most advanced application of mRNA in cancer immunotherapy is therapeutic vaccination. It leverages both the capability of mRNA to deliver genetic information and to stimulate the immune system. The latter is particularly important for breaking immune tolerance when cancer-associated self-antigens are targeted. Other applications of mRNA in cancer immunotherapy include the engineering of immune cells with modified antigen receptors, and its use as a template for immunologically active proteins in a variety of cells.
KnowMade has investigated the IP activity related to mRNA cancer treatments to understand the current developments and challenges the players face to fully unlock mRNA’s potential for this application. This report, published in September 2022 (available here: mRNA Cancer Therapies Patent Landscape 2022), identifies the main players for specific RNA types, RNA modification & stabilization, carriers, and different therapeutic modalities.
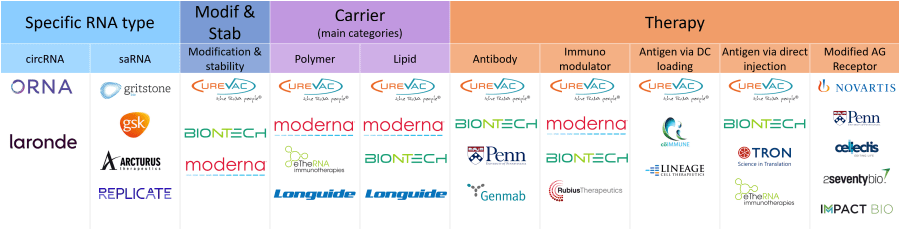
Involvement of some players in the different technologies described in the report.
Growing competition attesting to attractive technology
The IP landscape analysis shows growing interest and investment in the domain of mRNA cancer treatment possibilities as the number of new patent filings has constantly increased since 2014. The increasing patenting activity is driven by the main players (BioNTech, CureVac, Moderna) as well as the entrance of newcomers. These players, whether industrialists or academics, mainly have an international IP strategy. Moreover, the growing interest for this new technology is also visible through the large number of litigations. Indeed, there is a significant number of EP oppositions, reflecting the strategic issues of mRNA cancer therapies for assignees. Most of the proceedings are recent, filed in the past five years, and are still ongoing. Both dynamics attest to the attractiveness and interest of the industry for this new application.
Many remaining challenges need to be addressed
Today, players seem to focus their development on therapy methods. Indeed, around 80% of the patent corpus describes mRNA encoding specific proteins. For these cancer therapies, mRNA-encoded antigens via direct injection (i.e., vaccine) and mRNA-encoded modified antigen receptors (i.e., CAR-T) are the most studied options. However, for the moment, no antigen has been specifically targeted. Instead, mRNAs encoding for a multitude of antigens are described, such as KRAS, PSA, PSMA, MAGE, MUC1, Trp2, STEAP-1, HPV E6 or E7, CLDN6, NY-ESO-1, Kallikrein-2, PRAME, etc. Nevertheless, it is worth noting that a promising technology has emerged: the development of personalized cancer vaccines, which is in line with the rise of personalized medicine. These vaccines, created through neoepitope identification, are specific to the patient’s cancer (patient-specific cancer mutations).
Another big challenge is to find an efficient carrier to deliver the encoded protein. According to the patent analysis, polymers such as polyethylene glycol, poly-L-lysine, polyamidoamine, polyethyleneimine, chitosan, etc.; or lipids such as neutral or cationic lipids, liposomes, lipidoid, lipid nanoparticles; or a combination of polymers and lipids, are the most promising for mRNA cancer treatments. Lipid-derived and polymer-derived materials dramatically increased cellular uptake of RNAs, thus receiving tremendous attention in recent years.
Who will solve the last technological challenges and drive the emergence of a new generation of mRNA-based cancer treatments?
In 2022, this area is dominated by BioNTech (DE), CureVac (DE) and Moderna (US). Thanks to their strong background and know-how in the field, these players have successfully strengthened their IP position by covering the whole supply chain from mRNA (modification, stability and protein for which it codes) to carriers, as well as extending their inventions worldwide. Such broad technological and geographical coverage is testament to the critical importance of the IP for these players. Indeed, the COVID-19 experience (see KnowMade’s article on Moderna and Pfizer/BioNTech litigation – 2022) shows that these players are willing to aggressively defend their position by asserting their patents. In addition, the IP landscape reveals strong and growing competition. KnowMade has identified more than 50 IP newcomers since 2019, mainly from the US and Asia. Some of them are startup companies, with an incorporation date of 2017 or later, such as ImmPACT Bio, Replicate Bioscience or Nutcracker Therapeutics (see article on Nutcracker technology – 2022), while others are established companies such as eTheRNA Immunotherapies, Arcturus Therapeutics or Carisma Therapeutics. The entrance of these new players combined with the strong competition between IP leaders should lead to many patent litigations, as well as M&A to secure IP and market position, and accelerate players’ time to market.
KnowMade’s latest work on mRNA: mRNA cancer therapies.
All our reports on healthcare patent landscape.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About the author
Fabienne Massa works at KnowMade in the field of Biotechnology and Life Sciences. She holds a PhD in Molecular and Cellular Biology from the IPMC (Nice, France). She also holds a Master of Business Management from IAE (Nice, France) and she previously worked in the pharmaceutical industry.
About Knowmade
KnowMade is a Technology Intelligence and IP Strategy consulting company specialized in analysis of patents and scientific information. The company helps innovative companies and R&D organizations to understand their competitive landscape, follow technology trends, and find out opportunities and threats in terms of technology and patents.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patents and scientific information into business-oriented report for decision makers working in R&D, Innovation Strategy, Intellectual Property, and Marketing. Our experts provide prior art search, patent landscape analysis, scientific literature analysis, patent valuation, IP due diligence and freedom-to-operate analysis. In parallel the company proposes litigation/licensing support, technology scouting and IP/technology watch service.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Solid-State Sensors/Actuators, Semiconductor Manufacturing, Packaging & Assembly, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
