SOPHIA ANTIPOLIS, France – April 20, 2022 | During recent decades, biopharmaceuticals have gained considerable attention as revolutionary therapeutics and vaccine strategies, attempting to fulfil the high expectations of resolving serious health conditions. Regarding nucleic acids, mRNA-based technologies have been established as promising approaches in therapy and prevention of numerous diseases. mRNA’s great potential can manifest clinically through vaccination against infectious diseases (e.g., COVID-19) and cancers, protein replacement therapies, tissue regeneration and the treatment of genetic diseases.
Current available centralized production of mRNA therapeutics can be costly, slow, and susceptible to contamination. Development of scalable mRNA manufacturing, production of single patient dosages, elimination of touchpoints to limit contamination, input and process tracking for meeting clinical manufacturing requirements, and use in point-of-care operations can advance the use of these promising therapeutic modalities.
To meet these needs, Nutcracker Therapeutics, an American company co-founded in 2018 by Igor Khandros (founder of Berkeley Lights, FormFactor and Tessera Technologies) and Ben Eldridge, is developing mRNA-based therapeutic drug products which will be manufactured on its ACORN (Automation COntrolled RNA) microfluidic based manufacturing system. ACORN, as a microfactory, is a fully automated, software-controlled microfluidics platform. It combines RNA biochemistry with microfluidic engineering, semiconductor-like biochips, and a proprietary nanoparticle delivery technology to create a fully automated and isolated manufacturing pathway. Its GMP-in-a-box is expected to offer significant cost, cycle-time, capacity and point-of-care execution advantages over other RNA manufacturing approaches. These developments have enabled the company, in March 2022, to raise $167 million in Series C financing, led by ARCH Venture Partners. The funds will allow the company to expand and advance its pipeline of mRNA medicines for cancer, in addition to further refining its RNA manufacturing platform and the underlying technology.
However, very few details regarding the technologies developed are disclosed by Nutcracker Therapeutics. Indeed, the company’s communication is much more focused on operational modalities than on the technologies implemented in its product. Therefore, to fully understand the heart of these disruptive technologies, it is essential to analyze Nutcracker Therapeutics’ patent portfolio, which currently comprises 7 patent families. This patent portfolio can be split into 3 categories: mRNA carriers, Microfluidic devices, and mRNA therapeutics.
mRNA CARRIERS
Regarding carriers, Nutcracker Therapeutics filed its first patent application in 2019 (Tertiary amino lipidated cationic peptides for nucleic acid delivery – WO2020069442). This disclosure relates to multicomponent lipid compositions comprising lipidated cationic peptide compounds for the delivery of mRNA with improved efficiency. Tertiary amino lipidated and/or PEGylated cationic peptide is here N-substituted cationic peptide compounds that are N-substituted with lipid moieties and/or (oligo- and/or poly)ethylene glycol moieties. As for lipitoid, it is a cationic peptide-phospholipid conjugate (fig.1).
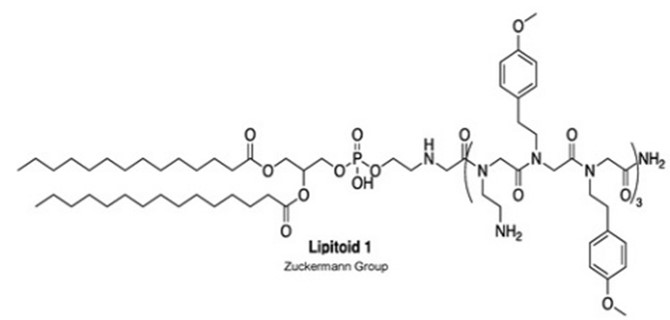
Figure 1: Exemplary secondary amine phospholipidated cationic peptide “Lipitoid 1”.
To demonstrate the efficacy of the compounds, an in vivo experiment was described. Amino lipidated peptoid compounds were evaluated for in vivo delivery efficiency of firefly luciferase (fluc) mRNA to Balb/c mice by subcutaneous injection administration. Multicomponent lipid formulations comprising amino lipidated peptoids with Fluc mRNA (cholesterol, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), and 2-dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000 (DMG-PEG2000) were prepared. The formulations were administered at a dose of 0.1 mg/kg via a tail-vein injection, and the resulting bioluminescence was quantified after 6 hours. The results show that the in vivo administration of the various compounds and the transcription of the luciferase work. Regarding this first patent family, a lot of pending patent applications were published (US, Europe, Asia etc.). According to the written opinion of the International Searching Authority (ISA), claim 1 meets the criteria of novelty and should be granted with some modifications at the end of the patent processes in these territories.
Moreover, in 2020, Nutcracker Therapeutics filed another patent application related to compositions of cationic compounds for the delivery of nucleic acids (Lipidated cationic peptide-peg compositions for nucleic acid delivery – WO2021030218). According to this invention, lipitoid, which has been widely evaluated as a standalone cationic delivery vehicle, still suffers from further complications in vivo, including, for example, particle instability and aggregation. Therefore, the invention provides tertiary amino lipidated and/or PEGylated peptoids, and complexes and compositions thereof having low concentrations of PEGylated lipids for nucleic acid delivery. It also provides methods for preparing complexes and compositions. To test these new compounds, an in vivo experiment has been carried out. Representative amino lipidated peptoid/Fluc formulations (prepared at a 5:1 mass ratio of amino lipidated peptoid:mRNA unless noted, with 2% w/w DMG-PEG2000) were administered at a dose of 0.5 mg/kg via a tail-vein injection, and the resulting bioluminescence was quantified after 8 hours (fig. 2). Results demonstrated the feasibility and the efficacy of the in vivo compounds administration. This patent family comprises pending applications in Europe and other territories. According to the written opinion of the ISA, claim 1, which is relatively specific, meets the criteria of novelty and should be granted with some modifications at the end of the patent processes in these territories.
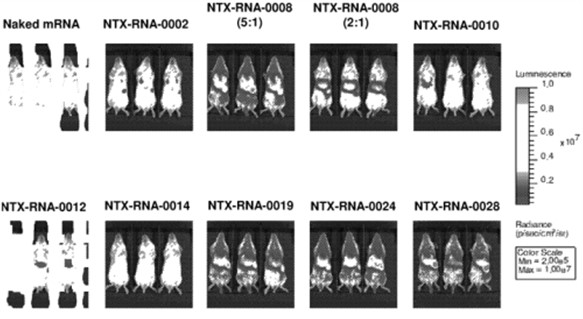
Figure 2: whole body distribution of the formulations in the Balb/c mice.
MICROFLUIDIC DEVICES
Regarding microfluidic devices, Nutcracker Therapeutics filed 2 patent families in 2020 with numerous foreign extensions (US, Europe, Asia). First, the company disclosed a patent family which described a microfluidic apparatus for processing polynucleotides in a sealed path environment (Microfluidic apparatus and methods of use thereof – US20210039106). The methods and apparatuses (microfluidic instrumentation and processes) described provide scalable polynucleotide manufacturing, production of single patient dosages, elimination of touchpoints to limit contamination, input and process tracking for meeting clinical manufacturing requirements, and use in point-of-care operations for therapeutics (fig. 3). According to the written opinion of the ISA, claim 1 meets the criteria of novelty and should be granted with some modifications at the end of the patent processes in the US, Europe, and Asia.
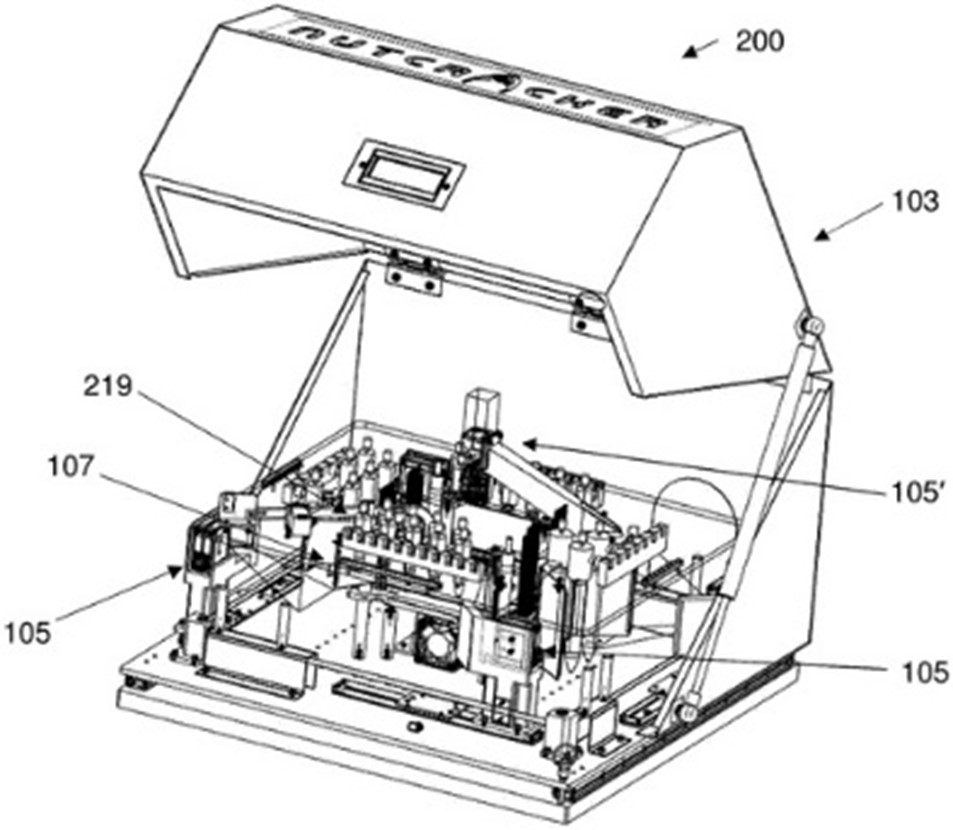
Figure 3: diagrammatic representation of one example of a system for processing polynucleotides.
Moreover, Nutcracker Therapeutics filed a patent family describing a microfluidic mixing chamber (or a series of interconnected microfluidic mixing chambers) that are configured to provide highly efficient mixing in a relatively small footprint (Mixing and microfluidic apparatuses related thereto – US11278895). These mixing chambers operate within a particular flow rate to achieve a high degree of mixing and include temperature control of the mixing chamber (fig. 4). The mixing channels can receive 2 or more fluids from the inlet comprising mRNA and a lipidated peptoid delivery vehicle. This patent family comprises pending patent applications in the US, Europe, and Asia, and also one granted patent in the US.
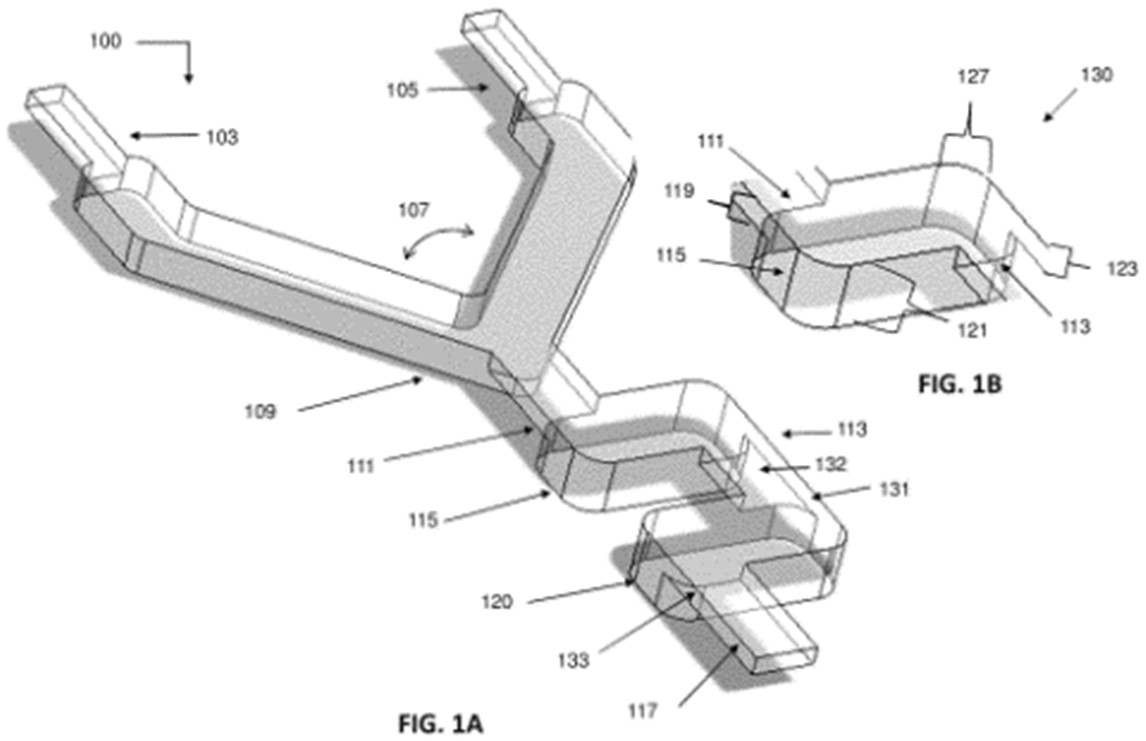
Figure 4: example of a microfluidic mixing apparatus and a microfluidic mixing unit.
mRNA THERAPEUTICS
In 2021, Nutcracker Therapeutics filed 2 patent families describing mRNA cancer therapeutics. Regarding patent application WO2021216647 (mRNA treatment nanoparticles), the treatment disclosed is an intratumoral injection of an mRNA nanoparticle encoding a tumor-specific antigen, and an mRNA encoding an immunomodulatory agent, wherein they are encapsulated together by an amino lipidated peptoid. To observe the treatment efficacy, an experiment was performed using a syngeneic mouse model in which a different tumor cell line (MC38) was used. The mRNA vaccine including tumor-specific antigens identified by sequencing the MC38 tumor and neo epitopes were identified. Seven epitopes were identified and chained to form a single mRNA encoding all seven tumor-specific antigens; the sequences of the epitopes were expressed in a custom scaffold to enhance presentation. In figure 5 showing Kaplan Meier Survival curves for a mouse tumor model, the control animals (“G2”) were injected with a non-coding mRNA and CpG, along with the amino lipidated peptoid delivery vehicle. Subcutaneous injection of the mRNA vaccine including just the tumor-specific mRNA and the amino lipidated peptoid delivery (“G7”) did not significantly impact tumor growth, as compared to control animals. In contrast, animals injected intratumorally with both the tumor specific mRNA and anti-CTLA-4 mRNA combined with an amino lipidated peptoid (“G9”) showed a significant slowing or reduction in tumor growth and therefore survival. Most significantly, intratumoral injection of an mRNA vaccine including an mRNA of the tumor-specific antigen and mRNA encoding three immunomodulatory agents (anti-CTLA-4, TGF-β and single chain IL-12) with an amino lipidated peptoid delivery vehicle (“G10” showing a “multi-effector cocktail”) resulted in a dramatic slowing or reduction in tumor growth. This patent family comprises a pending PCT patent application. However, according to the written opinion of the ISA, most claims do not meet the criteria of novelty and inventive step.
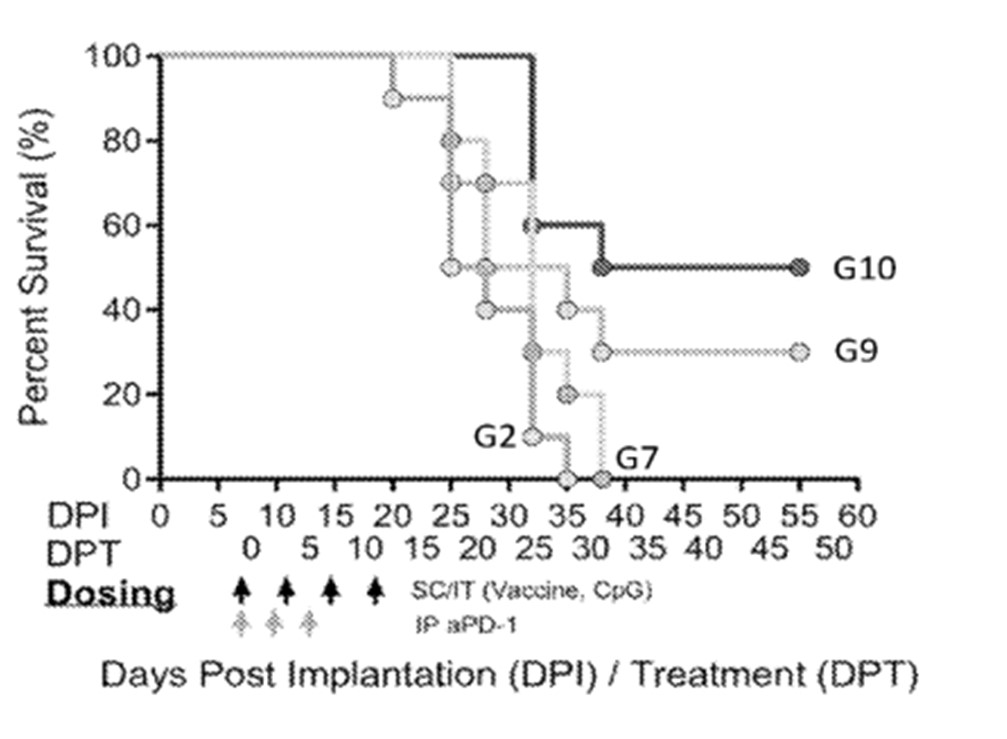
Figure 5: Kaplan Meier Survival curves for a mouse tumor model.
The second patent family related to cancer treatment described methods of preparing and using the multicomponent delivery systems, comprising lipidated peptoid components such as a lipidated cationic peptoid component, and additionally a non-cationic lipididated peptoid component (Multicomponent delivery systems for polyanionic cargo compound delivery – WO2022032058). According to the invention, when the multicomponent delivery systems are complexed with mRNA, they can be used as vaccines to elicit strong cellular and humoral immune responses. These delivery systems have the benefit of providing nanoparticles that are stable and monodisperse, and can be administered through a variety of routes (e.g., intravenous, intratumoral, subcutaneous, and intramuscular). The efficacy of multicomponent delivery systems in a disease model was evaluated by formulating mRNA coding for Ovalbumin (OVA) or with the disclosed peptoids and administering this vaccine to C57B1/6 mice. Vaccine candidates were administered twice, with a prime on Day 0 of the study and a boost on Day 7. The resulting immune response to characterized epitopes was determined on Day 14 by measuring levels of antigen-specific CD8+ T cells in peripheral blood and spleen. The multicomponent delivery vehicle systems of the disclosure elicited a strong T cell response. This recent patent family comprises a pending PCT patent application, and according to the written opinion of the ISA, no claim meets the criteria of novelty and inventive step, so these claims will have to be amended.
Another patent family filed by the company, not specific to cancer therapy, provides examples related to polynucleotides, scaffolds, and cassettes (Polynucleotides comprising an antigenic payload – WO2021231541). This disclosure describes examples related to fusion molecules which comprise polypeptide antigens (e.g., tumor antigens, neoantigens, infectious agent antigens), engineered as a payload incorporated into a scaffold where such scaffold comprises regions of a parental receptor molecule (e.g., signal sequence, extracellular region, transmembrane region and/or cytoplasmic region), for antigen presentation at the surface of a cell or at a specific cellular compartment. These polynucleotides and scaffolds can be used for many applications, including inducing an immune or therapeutic response. An ex-vivo stimulation in a healthy donor has been carried out. Cryopreserved Human Cytomegaly Virus (CMV) seropositive Healthy Donor (CMV+) Peripheral blood mononuclear cells were treated with either 1 µg mRNA encoding pp65 with Sec-hCDld MHC presentation enhancing sequences, 2µM CMV pp65 peptide pool covering the entire pp65 molecule, or non-coding mRNA. After 6 days, cells were harvested and CD8 T cells were isolated. Viability was measured, cells were counted and harvested. HLA-A2:01 expressing T2 cells were then labeled with cell tracer violet dye. Half of the T2 cells were pulsed, while the other half were left un-pulsed, with CMV pp65 peptide. Then, pulsed or un-pulsed T2 cells were added to the isolated CD8 T cells and co-incubated. CD8 mediated T2 killing was analyzed by flow cytometry (fig. 6).
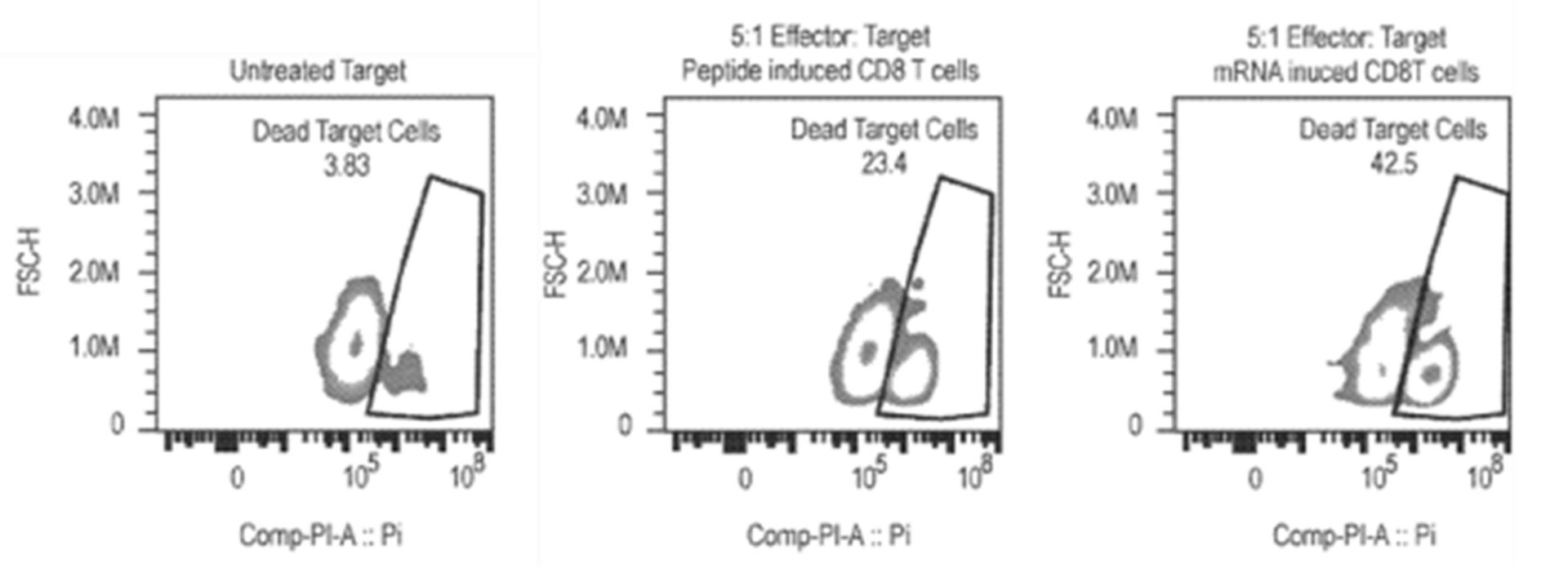
Figure 6: flow cytometry results for untreated target cells, peptide induced CD8 T cells, and mRNA induced CD8 T cells.
Results showed that after 6 days of passive expansion, robust CD8 T cell growth in cultures treated with 1 µg mRNA encoding pp65 with Sec-hCDld was observed. Both viability and cell numbers were superior compared to cells treated with peptide. The activated and expanded CD8 T cells were able to recognize and kill a T2 target cell only when the T2 target cell was pulsed with CMV-pp65 antigens. Significantly better killing efficacy in CD8 T cells isolated from cultures that were treated with mRNA compared to peptides was observed (fig. 7). These results show that large numbers of functional (i.e., able to kill target cells) CD8 T cells can be generated by treating whole PBMC populations with nanoparticles containing mRNAs encoding antigens and an MHC trafficking signal Sec-hCDld. This recently patent family currently comprises a pending PCT patent application. According to the written opinion of the ISA, the first claims meet the criteria of novelty but not those of inventive step.
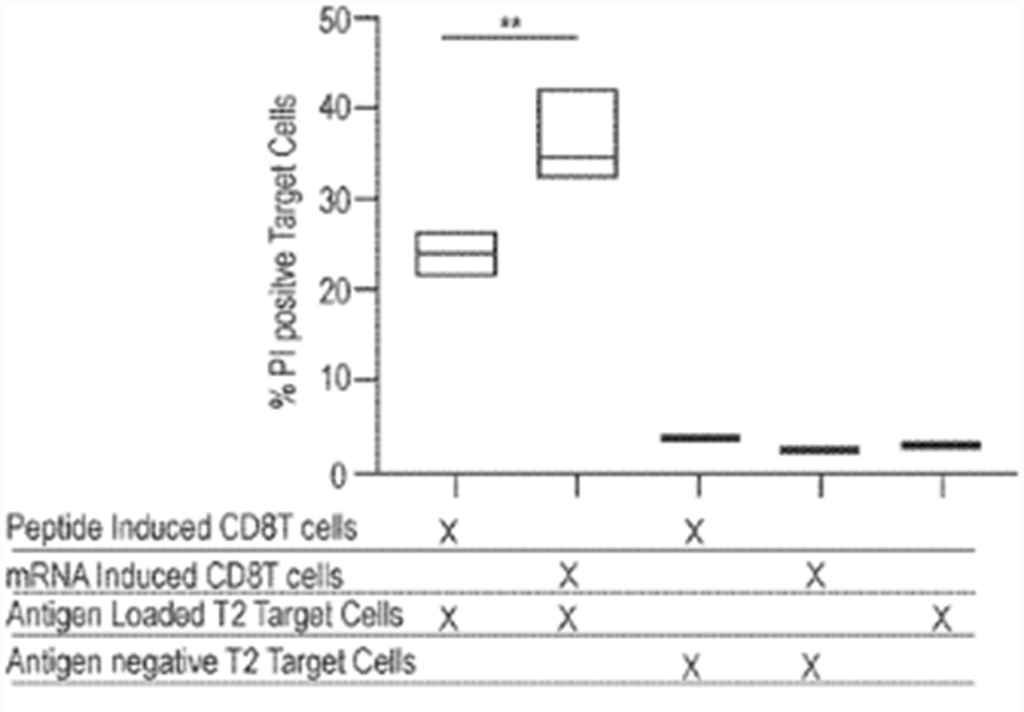
Figure 7: comparison of % PI positive target cells for samples of induced CD8 T cells.
To conclude, Nutcracker Therapeutics is developing mRNA-based therapeutic drug products manufactured in its proprietary microfluidic system. Its patent portfolio, comprising 7 patent families, discloses an amino lipidated peptoid delivery vehicle that encapsulates mRNA, point-of-care microfluidic systems to manufacture mRNA therapeutics, especially by mixing mRNA with a vehicle delivery system, and mRNA therapeutics for treating cancer and other conditions. In this way, its patent portfolio covers the production of therapeutic mRNA via its microfluidic device, and the use thereof. Most of its patent families comprise pending patent applications, and several granted patents in the US, Europe and Asia are expected next year, strengthening Nutcracker Therapeutics’ position in the field of personalized mRNA therapeutics delivered at the point of care.
Life sciences patent reports by KnowMade.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About our analysts
Fabienne works at Knowmade in the field of Biotechnology and Life Sciences. She holds a PhD in Molecular and Cellular Biology from the IPMC (Nice, France). She also holds a Master of Business Management from IAE (Nice, France) and she previously worked in the pharmaceutical industry.
About Knowmade
Knowmade is a Technology Intelligence and IP Strategy consulting company specialized in analysis of patents and scientific information. The company helps innovative companies and R&D organizations to understand their competitive landscape, follow technology trends, and find out opportunities and threats in terms of technology and patents.
Knowmade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patents and scientific information into business-oriented report for decision makers working in R&D, Innovation Strategy, Intellectual Property, and Marketing. Our experts provide prior art search, patent landscape analysis, scientific literature analysis, patent valuation, IP due diligence and freedom-to-operate analysis. In parallel the company proposes litigation/licensing support, technology scouting and IP/technology watch service.
Knowmade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Solid-State Sensors/Actuators, Semiconductor Manufacturing, Packaging & Assembly, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
