SOPHIA ANTIPOLIS, France – November 06, 2023 │ReNAgadeTherapeutics, a company founded in 2021 and based in Cambridge, presented preclinical data demonstrating a new and proprietary method of delivering mRNA to extra-hepatic tissues in non-human primate using lipid nanoparticles (LNPs) at the 11th International mRNA Health Conference, taking place from October 31 to November 2, 2023 in Berlin, Germany.
ReNAgade Therapeutic, a promising LNP biotech
Certain pharmaceutical compositions, such as lipid nanoparticles, have been found to effectively protect nucleic acids and facilitate their safe delivery to cells. However, targeting a specific organ with these nucleic acids remains a challenge, especially avoiding main delivery to the liver. In this objective, ReNAgade is building a toolbox to enable various RNA medicines. In particular, thanks to the LNP engineering and screening platform ReNAgade has developed a portfolio of novel LNPs and identified extra-hepatic LNPs. Results presented last week, validate the delivery into non-hepatic cells type (NK cells and HXCs) of several of extra-hepatic LNP’s in non-human primate and humanized mouse model. From a technical standpoint, it is interesting to understand the composition of its proprietary lipid nanoparticle (LNP)-based delivery of mRNA. To do so, analyzing the patent portfolio of the company provides valuable insight. Currently, ReNAgade Therapeutics has 10 patent families, published between December 2022 and October 2023.
Insight into RenAgade’s technology: Analysis of its 1st patent application
The first patent application filed by the company (WO2022/251665) relates to pharmaceutical compositions containing lipid nanoparticles capable of delivering polynucleotide payloads to non-liver target organs. This patent application claims a pharmaceutical composition designed for significant extrahepatic delivery, consisting of: a) a lipid nanoparticle containing at least one ionizable lipid, and b) a polynucleotide; where at least 5% of the polynucleotide is delivered to a target organ other than the liver.
Chemical structure of ionizable lipids for LNP-mediated mRNA delivery to non-hepatic tissues
This invention mainly focuses on two ionizable lipids for LNP-mediated delivery to non-hepatic tissues, compound 1 and compound 2, illustrated in Figure 1 below. The synthesis of these 2 ionizable lipids is described in this patent application.
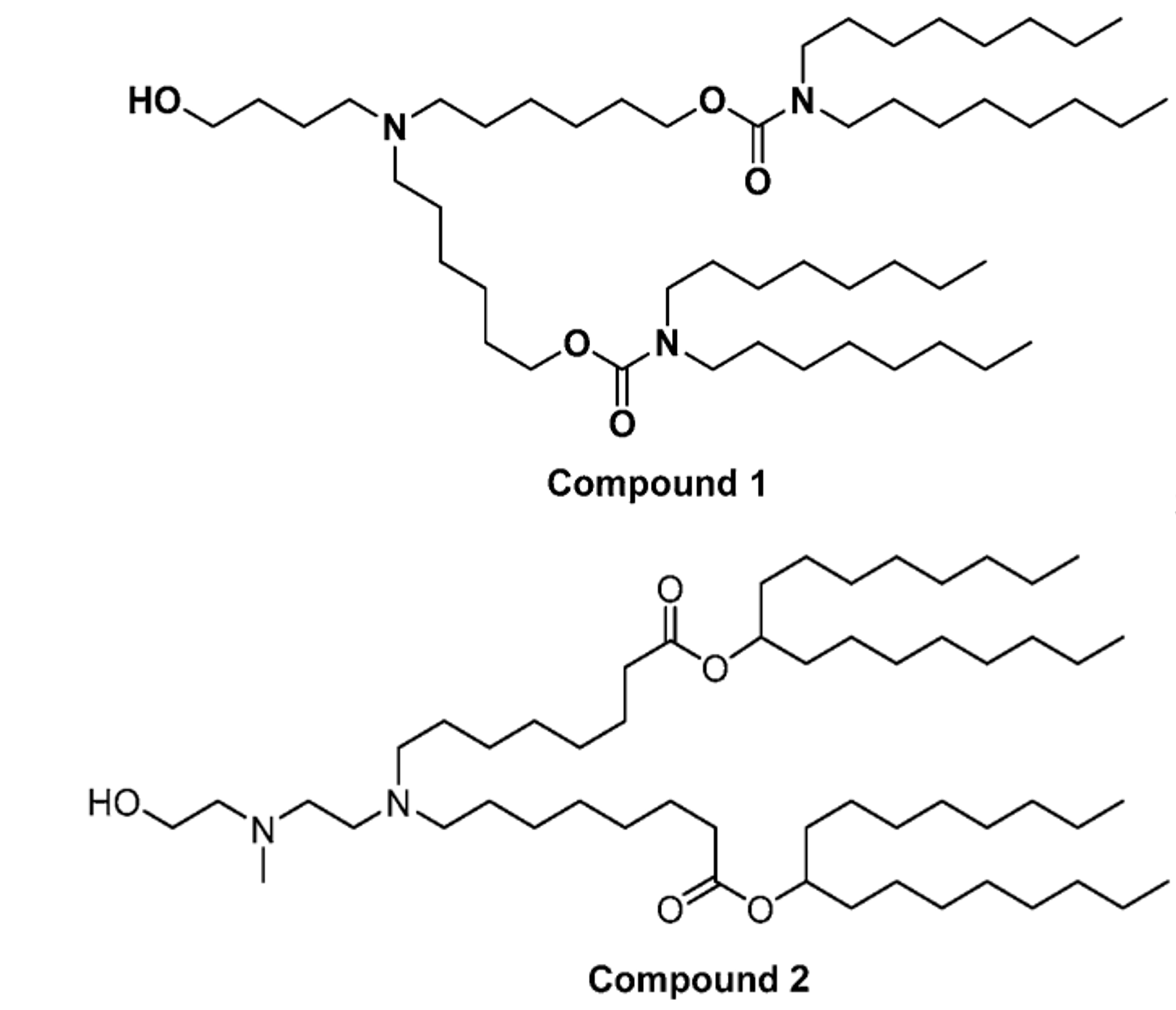
Figure 1: Structure of ionizable lipids.
Compound 1: ((4-hydroxybutyl) azanediyl) bis(hexane-6,1-diyl) bis(dioctylcarbamate)
Compound 2: heptadecan-9-yl 8-((2-((2- hydroxyethyl)(methyl)amino)ethyl)amino)octanoate (L2-4) and di(heptadecan-9-yl) 8,8′-((2-((2- hydroxyethyl)(methyl)amino)ethyl)azanediyl)dioctanoate
LNP formulations
In the examples of the description, several ionizable lipids are synthesized for the preparation of LNPs. Formulations F-1, F-2, and F-6, highlighted in green in the figure 2 below, correspond to LNPs containing the ionizable lipids of the invention. Compounds 3 and 4 are also used as comparative ionizable lipids. Compound 4 was synthesized based on the work of Lokugamage et al. in 2019, while Compound 3 was synthesized based on the work of Zaho et al. in 2020.
In detail, ionizable lipids, phospholipids, cholesterol, and PEG-lipids were dissolved in pure ethanol at the ratios indicated in Figure 2 below, with a total lipid concentration of 10.8 mM. (Qiu et al., 2021). The lipid solution was then mixed with an acidic sodium acetate buffer (pH 5.0) or sodium citrate buffer (pH 4.0) containing mRNA (0.10 mg/mL) at a 3:1 volume ratio using the NanoAssemblr microfluidic system, with a total flow rate of 12 mL/min to achieve rapid mixing and self-assembly of LNPs. The resulting formulations were further dialyzed against PBS (pH 7.4) overnight at 4°C. Each of the formulations F-1 through F-7 were then used to encapsulate batches of linear firefly luciferase (fLuc) mRNA or green fluorescent protein (GFP) mRNA.
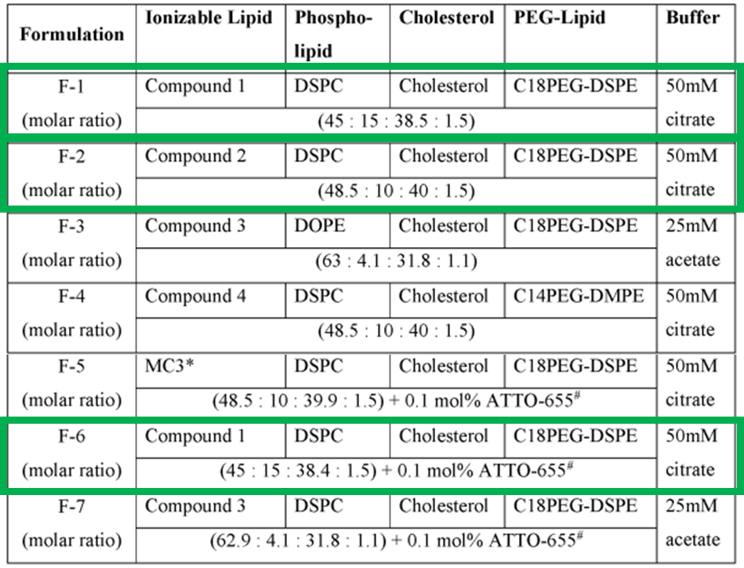
Figure 2: LNP Formulations.
The formulations F-1, F-2, and F-6, highlighted in green in the table, correspond to the LNPs incorporating the ionizable lipids of the invention.
*MC3 = 4-(dimethylamino)-butanoic acid, (10Z,13Z)-1-(9Z,12Z)-9,12-octadecadien-1-yl-10,13- nonadecadien-1-yl ester; #ATTO-655 = Sigma Aldrich product no.93711
In Vivo FLuc mRNA Delivery and Bioluminescence Measurements
BALB/c mice were injected with 1.0 mg/kg FLuc mRNA via the tail vein in LNP formulations F-1, F-2, F-3, and F-4 with a total volume of 5mL/kg. The main outcome of this example is that formulations F-1 and F-2 demonstrated better extra-hepatic distribution and higher efficiency for delivering mRNA (FLuc) than the previously published formulations F-3 and F-4, specialized in extra-hepatic delivery (see Figure 3). This suggests that formulations F-1 and F-2 may be promising options for targeted delivery of mRNA outside of the liver.
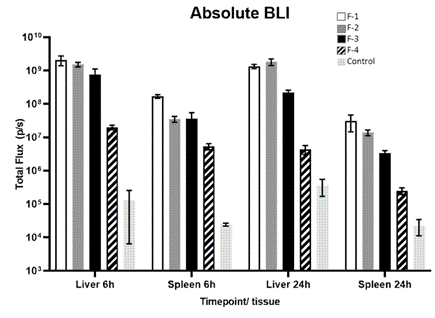
Figure 3: Absolute luminescence of fLuc mRNA in liver and spleen of mice dosed with fLuc mRNA formulated within LNP formulations F-1, F-2, F-3, F-4 and PBS control, 6 hours and 24 hours after administration.
Additional in vivo FLuc mRNA Delivery and Bioluminescence Measurements
In an additional experiment, BALB/c mice received an injection of 1.0 mg/kg FLuc through the tail vein, which was formulated with either LNP formulations F-1 or F-3 in a total volume of 5mL/kg. Figure 4 displays a quantitative comparison of the distribution of Formulations F-1 and F-3 in the liver and spleen of the mice following a single IV administration of fLuc mRNA LNPs, as determined by bioluminescence imaging. These results support previous findings that LNP formulation F-1 is more effective for delivering a cargo mRNA to extraparenchymal tissues, compared to previously published LNP formulations designed for extraparenchymal delivery.
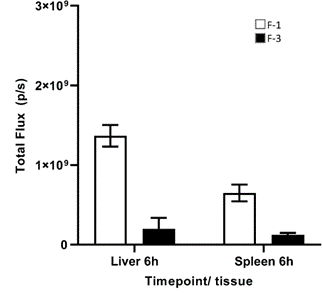
Figure 4: Absolute luminescence of fLuc mRNA in liver and spleen of mice dosed with fLuc mRNA formulated within LNP formulations F-1 and F-3, 6 hours after administration.
Flow Cytometry Analysis of Splenocytes
BALB/c mice received tail vein injections of GFP mRNA formulated in LNP formulations F-5 (2.0 mg/kg GFP mRNA, comparative example), F-6 (1.5 mg/kg GFP mRNA, containing ionizable lipid compound 1 from this invention) and F-7 (1.5 mg/kg GFP mRNA, comparative example) in a total volume of 5mL/kg. Significant GFP expression was detected in red pulp macrophages, CD11b+ IA/E+ myeloid cells, and dendritic cells among the examined spleen cell types. B cells and neutrophils did not show substantial GFP expression. Bar graphs in Figures 5 A-C show GFP expression levels in these cell types 1 hour after administration. F-6, which contained Compound 1, the ionizable lipid of the invention, demonstrated the highest GFP expression in the tested cells.
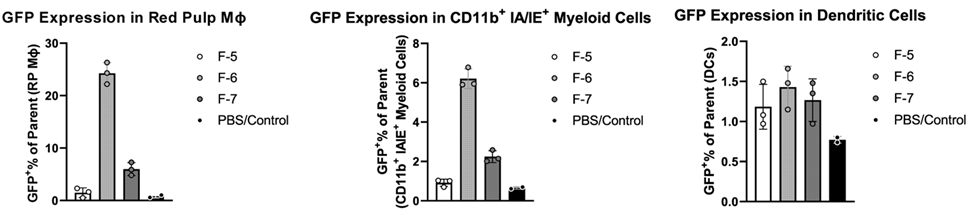
Figure 5: GFP expression by flow cytometry in red pulp macrophages (FIG.5A), CD11b+ IA/IE+ myeloid cells (FIG.5B), and dendritic cells (FIG.5C), 1 hour after administration.
Legal analysis of the patent application
Regarding the patent application WO2022/251665, according to the search report, no document of particular relevance was identified, and the written opinion of the International Searching Authority (ISA/US) indicates that claim 1 (the only independent claim in this patent application) is novel and involves an inventive step. In addition, a European patent application has been filed and should be published soon. Based on the written opinion of the ISA, it is highly likely that claim 1 will be granted without amendments. Furthermore, there may still be possibilities for extensions in foreign countries, such as the US or Asia.
ReNAgade therapeutic’s IP strengthness
In summary, ReNAgade Therapeutics disclosed in this patent application ionizable lipids enabling targeted delivery of mRNA outside of the liver, particularly to the spleen or myeloid cells, and it is likely that the company will obtain IP protection covering its proprietary lipids with a wide geographical coverage. In addition, the results in non-human primate recently presented confirm the efficient delivery in non-hepatic cells such as NK cells and HSPCs that enable to solve current medical application due to poor delivery.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
About the author
Brice Sagot, CEO and co-founder of KnowMade, leads the Biotechnology and Life Sciences department. He holds a PhD in Molecular Biology from the University of Nice SophiaAntipolis (France).
Elodie Bovier, PhD., works at KnowMade as a Patent Analyst in the field of Biotechnology and Life Sciences. She holds a PhD in genetic and molecular biology from the Paris Sud University. She also holds the Industrial Property International Studies Diploma (in Patent and Trademark & Design Law) from the CEIPI (Strasbourg, France).
About KnowMade
KnowMade is a technology intelligence and IP strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patent information and scientific literature into actionable insights, providing high added value reports for decision makers working in R&D, innovation strategy, intellectual property, and marketing. Our experts provide prior art search, patent landscape analysis, freedom-to-operate analysis, IP due diligence, and monitoring services.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Sensors, Semiconductor Packaging, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
