SOPHIA ANTIPOLIS, France – October 17, 2025 │ This quarter, KnowMade’s Therapeutic mRNA patent monitoring service reveals two emerging players shaping the next wave of RNA innovation: Parcel Bio, pioneering nanoparticle-free delivery, and ArkeaBio, developing mRNA vaccines for methane mitigation.
Two Emerging Players Expand the Frontiers of Therapeutic mRNA Innovation
Two new entrants have emerged in the therapeutic mRNA patent landscape this quarter, each showcasing the diversity and dynamism of innovation within the field. Parcel Bio is pioneering a novel nanoparticle-free RNA delivery system designed to overcome the limitations of lipid nanoparticles (LNPs), while ArkeaBio applies mRNA technology in an entirely different arena, aiming to reduce agricultural methane emissions through microbial immunization. Their distinct approaches illustrate how the mRNA field continues to evolve beyond traditional human therapeutics, expanding into both technological and environmental applications that push the boundaries of RNA medicine.
Parcel Bio: Reinventing RNA Delivery Beyond Nanoparticles
A Newcomer Redefining the Rules of RNA Delivery
Parcel Bio, a San Francisco biotechnology startup founded in 2023, has entered the KnowMade’s Therapeutic mRNA patent monitoring service with its first patent application published on August 7, 2025. Parcel Bio is a seed-stage, venture-backed company, with David Weinberg, PhD (CEO) and Chris Carlson, PhD (CSO) as co-founders. Parcel Bio aims to disrupt the RNA delivery space by addressing a long-standing limitation: the reliance on lipid nanoparticles (LNPs) for systemic mRNA delivery.
Despite major breakthroughs in mRNA design and manufacturing, efficient, safe, and targeted delivery remains the key technological challenge. LNPs, the current gold standard, are associated with immunogenicity, limited tissue targeting, rapid hepatic clearance, and dose-limiting toxicity, particularly in repeated dosing scenarios.
STAmP™: A Nanoparticle-Free Path to Safe and Targeted mRNA Delivery
Parcel Bio’s proprietary STAmP™ platform, short for Stabilization and Targeting by Annealing mRNA to ParcelOligos, is a non-viral, nanoparticle-free delivery system for mRNA medicines. Instead of encapsulating mRNA in lipid nanoparticles, the STAmP approach uses short, chemically modified synthetic oligonucleotides, known as ParcelOligos, to tile the length of the mRNA molecule. These oligos not only protect the transcript from enzymatic degradation but also allow targeted delivery to specific cell types through conjugation with ligands such as GalNAc or C16.
Based on the patent application WO2025/166052, the claimed advantages of this technology comprise enhanced stability and reduced immunogenicity as illustrated in figure 1. Indeed, ParcelOligo-complexed mRNA resists degradation in serum (Example 1) and withstands RNase digestion (supported by examples 4, 7) and chemically modified oligos (e.g., 2′-OMe, 2′-MOE, C16) do not trigger innate immune responses, enabling repeated dosing (Supported by examples 2, 5, 8). Other advantages claimed are:
- Efficient translation: Complexed mRNA remains functional and translates efficiently into protein both in vitro and in vivo (Supported by examples 6, 9–12).
- Targeted delivery: In vivo experiments show that STAmP complexes can direct expression to the spinal cord (example 11) or liver (example 13), depending on the targeting ligand used.
- Carrier-free uptake: C16- or GalNAc-conjugated oligos facilitate transfection without lipid nanoparticles or transfection reagents, even in primary cells (Example 13).
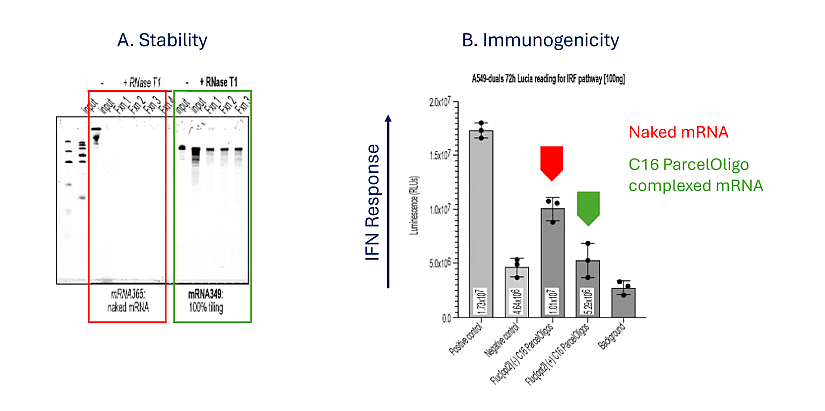
Figure 1: Enhanced stability and reduced immunogenicity.
A. Complexing Fluc[opt2] mRNA with 9 ParcelOligos spanning the CDS and 3’ UTR (in green) protects the mRNA from enzymatic degradation by RNase Tl, as indicated by the presence of the lower bands in the (+) lane. The higher bands present in both the untreated (-) and treated (+) lanes represent the poly(A) tail which is not degraded by RNase Tl, due to the lack of guanosine nucleotides
B. Naked Fluc[opt2] mRNA (red arrow) elicited an IFN response as indicated by the induction of luminescence signal (le7 RLU).
C16 ParcelOligo complexed Fluc[opt2] mRNA (green arrow) demonstrated a level of luminescence equivalent to the negative control.
These data indicate that complexing C16 ParcelOligos to the CDS and 3’ UTR of the mRNA aids in innate immune evasion. Figure adapted from patent publication.
ArkeaBio’s Nucleic Acid Vaccines Target Methanogens to Cut Livestock Emissions
From Lab to Pasture: ArkeaBio’s mRNA Approach to Greener Agriculture
ArkeaBio is a pioneering biotechnology company based in Boston, Massachusetts (USA), dedicated to combating agricultural methane emissions, one of the most potent contributors to climate change. The company develops innovative microbial-based vaccines that target methane-producing microbes in livestock, particularly in ruminants like cows, aiming to dramatically reduce enteric fermentation emissions.
What sets ArkeaBio apart is its strategic application of cutting-edge mRNA technology. By leveraging the principles of mRNA immunization, similar to those employed in human vaccines, the company designs microbial interventions that activate the animal’s immune system against methanogenic archaea in the rumen. This approach offers a scalable, low-carbon, and non-GMO solution to mitigate one of agriculture’s most stubborn environmental challenges.
With a mission aligned with global climate goals, ArkeaBio’s platform stands at the intersection of synthetic biology, microbiome science, and RNA therapeutics. The company represents a new frontier in sustainable agriculture, one where vaccine innovation can transform livestock from a major source of emissions into part of the climate solution.
ArkeaBio’s mRNA Platform: Targeting Methanogens to Cut Emissions at the Source
The general concept of ArkeaBio’s invention is a nucleic acid-based vaccine designed to generate an immune response against methanogenic archaea, microorganisms responsible for methane and hydrogen production in the digestive tract or other environments, as illustrated in the figure 2.
The vaccines encode antigens derived from methanogens, aiming to:
- reduce methane and/or hydrogen production;
- modulate the intestinal microbiome;
- treat or prevent diseases associated with excessive methanogen activity.
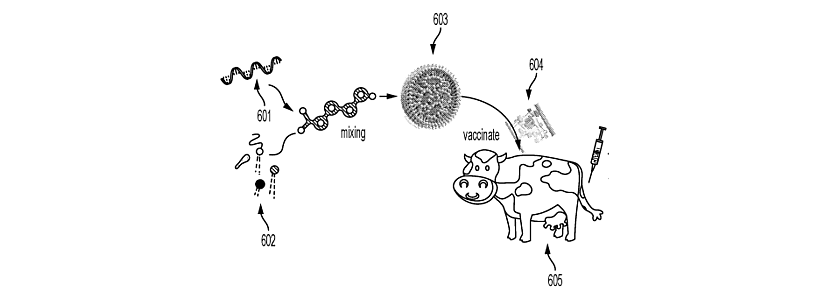
Figure 2: General principle of nucleic acid vaccines targeting methanogens
Nucleic acids, e.g., mRNA, (601) are mixed with lipids (602) to form lipid nanoparticles (603). The lipid nanoparticles (603) are formulated into a vaccine (604) and used to vaccinate a ruminant (605), such as a cow. Adapted from patent application WO2025/151711.
The description of WO2025/151711 identifies a broad range of methanogenic archaea as vaccine targets, primarily belonging to the orders Methanobacteriales, Methanomassiliicoccales, Methanosarcinales, and Methanomicrobiales. These taxa represent the major methane-producing archaea found in human and animal digestive ecosystems. Among them, the experimental section specifically evaluates antigens from Methanobrevibacter smithii, Methanobrevibacter ruminantium, Methanosphaera stadtmanae, and Methanomassiliicoccus luminyensis, demonstrating that vaccination with nucleic acid constructs encoding key enzymes (such as McrA and HdrA) elicits immune responses capable of reducing methane production in vitro and in vivo.
To support the concept, in Examples 1 and 2 of WO2025/151711, the inventors demonstrate the construction, expression, and immunogenicity of nucleic acid vaccines targeting Methanobrevibacter smithii. In Example 1, mRNA constructs encoding key methanogen antigens (specifically the methyl-coenzyme M reductase subunit A (McrA), the heterodisulfide reductase subunit A (HdrA), and a predicted surface glycoprotein) were synthesized from codon-optimized DNA templates under a T7 promoter. When transfected into HEK293 cells, these mRNAs yielded strong protein expression verified by Western blot and immunofluorescence, confirming proper translation and localization. Example 2 evaluated the same constructs formulated in LNPs and administered intramuscularly to BALB/c mice in a prime–boost regimen. Vaccinated mice developed robust systemic IgG and mucosal IgA responses against the recombinant methanogen antigens, with sera recognizing native proteins in M. smithii and Methanosphaera stadtmanae lysates. These findings establish both the effective in-cell expression of methanogen antigens and their capacity to elicit specific, cross-reactive immune responses in vivo.
Conclusion
The emergence of Parcel Bio and ArkeaBio in Q2–Q3 2025 underscores the remarkable breadth and momentum of innovation in the mRNA field. From delivery breakthroughs that promise safer, more targeted, and repeatable dosing, to novel applications transforming how we approach global challenges like methane emissions, these newcomers exemplify the sector’s rapid diversification.
KnowMade’s patent monitoring continues to capture these pivotal developments, offering strategic insights into how next-generation RNA technologies are shaping the future of therapeutics, biotechnology, and sustainability. For more detailed insights into these developments and how they may impact your business, please contact us.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About the author
Elodie Bovier, PhD., works at KnowMade as a Patent Analyst in the field of Biotechnology and Life Sciences. She holds a PhD in genetic and molecular biology from the Paris Sud University. She also holds the Industrial Property International Studies Diploma (in Patent and Trademark & Design Law) from the CEIPI (Strasbourg, France).
About KnowMade
KnowMade is a technology intelligence and IP strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patent information and scientific literature into actionable insights, providing high added value reports for decision makers working in R&D, innovation strategy, intellectual property, and marketing. Our experts provide prior art search, patent landscape analysis, freedom-to-operate analysis, IP due diligence, and monitoring services.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Sensors, Semiconductor Packaging, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.cahue
