SOPHIA ANTIPOLIS, France – September 28, 2023 │Since the FDA approval of two CAR-T cell therapies in 2017, CAR therapy has gained a big interest. Indeed, KYMRIAH™ (tisagenlecleucel) from Novartis and YESCARTA™ (Axicabtagene Ciloleucel) from Kite Pharma, a Gilead company, received the first FDA approval for chimeric antigen receptor (CAR) therapy in 2017. These two treatments are CD19-directed genetically modified autologous (patient-derived) T cell immunotherapies. Since then, many companies take the plunge and try to make improvements in CAR area such as structure modifications of CAR, the use of allogeneic (from healthy donor) CAR or even by trying to improve the manufacturing process of autologous CAR to make it faster.
KnowMade has investigated the IP activity related to allogeneic CAR to understand the advantage of allogeneic CAR on the autologous CAR such as price, speed of manufacturing and large-scale industrialization; and to understand the current developments and challenges that face the players to overcome graft-versus-host disease (GVHD) which is the major risk of allogeneic therapy. This report, published in September 2023 (available here: Allogeneic CAR Patent Landscape 2023), identifies main players for specific immune cell used, source of these immune cells, gene editing tools, and different therapeutic modalities.
A promising new technology in cell & gene therapies area
The IP landscape analysis shows that allogeneic CAR is a new technology in cell & gene therapies area. Currently, there are few patent families (416), but a growing interest is observed in the domain as the number of new patent families published has really increased since 2019. Moreover, the number of pending applications is very large and reveals the recent and strong interest in allogeneic CAR. Then, this sudden enthusiasm is also visible through the few numbers of EP opposition and the absence, for now, of patent litigation. The increasing patenting activity is driven by the main players (Cellectis, CRISPR Therapeutics and Fate Therapeutics) as well as the entrance of newcomers.
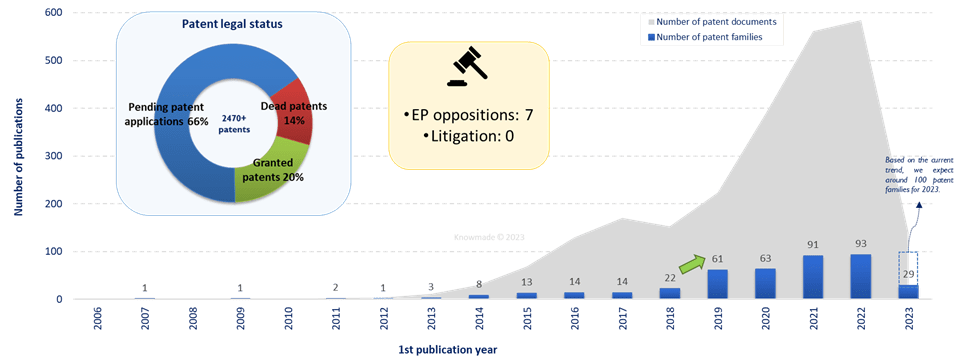
Figure 1: Timeline of patent publications and patent legal status of patent families related to allogeneic CAR
Many challenges to overcome GVHD
Graft-versus-host disease (GVHD) is the major risk of allogeneic CAR therapy. In GVHD, there is a cytotoxic alloreactivity of donor T cells which destroy tissues in the recipient. GVHD occurs due to immuno-incompatibility, e.g., human leukocyte antigen (HLA) mismatches between the donor and recipient. To overcome this problem, several solutions are proposed such as the use of non αβ T cell (e.g., NK cells, NK T cells or macrophages), the use of induced pluripotent stem cells (iPSC) or umbilical cord blood as source of immune cells or making gene editing to delete the αβ T cell receptor (TCR), for example. These technical solutions were used to segment the patent corpus selected in the report Allogeneic CAR Patent Landscape 2023.
Who will solve the main technological challenges and drive the emergence of an allogeneic CAR treatment?
In 2023, this area is dominated by Cellectis, CRISPR Therapeutics and Fate Therapeutics which adopt a worldwide IP strategy and extend their inventions in all countries which attests of the critical importance of the IP for these players. Cellectis shows a strong IP leadership and has a high blocking prior art potential (with a lot of granted patents which received many forward citations). Regarding CRISPR Therapeutics and Fate Therapeutics, they are strong IP challengers with their numerous pending applications. In addition, the IP landscape attests of a growing competition. KnowMade has identified more than 30 IP newcomers since 2019 coming mainly from the US and Asia. Some of them are startup companies, such as Artiva Biotherapeutics, Wugen or Sana Biotechnology, while other are established companies such as Caribou Biosciences, Poseida Therapeutics or Nkarta. These IP newcomers are very active in allogeneic CAR area. Indeed, some of them already have obtained FDA approvals such as Fast Track Designation or Investigational New Drug Applications while other collaborate with big pharmaceutical companies such as Bristol Myers Squibb or Roche. All of this information is compiled in the Allogeneic CAR Patent Landscape 2023 report to help understand and anticipate these developments in the field of immune-oncology and cell therapies.
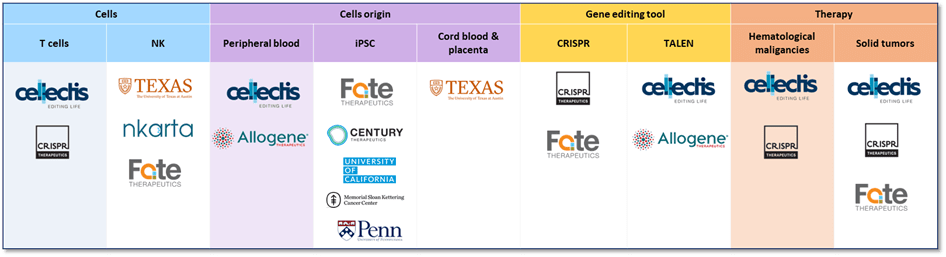
Figure 2: Involvement of some selected IP players in the different technologies related to allogeneic CAR
If you need more information, reach us at contact@knowmade.fr or with our contact forms.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Sophia Antipolis, France
www.knowmade.com
About our analyst
Fabienne Massa works at KnowMade in the field of Biotechnology and Life Sciences. She holds a PhD in Molecular and Cellular Biology from the IPMC (Nice, France). She also holds a Master of Business Management from IAE (Nice, France) and she previously worked in the pharmaceutical industry.
About KnowMade
KnowMade is a Technology Intelligence and IP Strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand their competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patent information and scientific literature into actionable insights, providing high added value reports for decision makers working in R&D, innovation strategy, intellectual property, and marketing. Our experts provide prior art search, patent landscape analysis, freedom-to-operate analysis, IP due diligence, and monitoring services.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Sensors, Semiconductor Packaging, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
