SOPHIA ANTIPOLIS, France – August 03, 2023 │ Today, immunotherapy is essential in cancer treatment. This field is therefore in full expansion and a lot of innovative therapies are emerging. In 2017, the FDA approval of two CAR-T cells therapies (KYMRIAH™ from Novartis and YESCARTA™ from Kite Pharma, a Gilead company) has enable the development of chimeric antigen receptor (CAR) technology. As happening with any treatment, side effects have been observed in these autologous (patient-derived) CAR therapies. To overcome these problems, several tracks are being studied, such as the use of allogeneic cells coming from a healthy donor. However, new complications are emerging, such as graft versus host disease (GVHD) which remains the major risk in allogeneic therapy. To avoid GVHD, many solutions are evaluated by companies and academics specialized in allogeneic CAR area. Among these solutions, the use of natural killer (NK) cells instead of T cells appears promising to reduce the risk of GVHD. That is the technology Nkarta has been focusing on, and the company is almost the only one.
Nkarta, an American biopharmaceutical company, was founded in 2015 on the proprietary and well characterized Natural Killer cell expansion technology pioneered by Dario Campana during his research at the St. Jude Research Hospital. Director of the Division of Immunopathology and Cell Therapy at the National University of Singapore, he is the scientific founder of Unum Therapeutics, whose name changed to Cogent Biosciences (specialized in the treatment of genetically driven diseases) and Medisix Therapeutics (specialized in cellular therapies for T cell malignancies). He was a member of the medical advisory board at Cellectis, a major player in allogeneic CAR area.
Nkarta is advancing the development of allogeneic, off-the-shelf NK cell therapies to treat cancer. Its approach for cellular immunotherapy involves CAR on the surface of a NK cell that enables the cell to recognize specific proteins or antigens present on the surface of tumor cells. According to the company, “all the product candidates are designed to be allogeneic, meaning they are produced using cells from a different person than the patient treated, as well as off-the-shelf, meaning they are produced in quantity, then frozen and therefore available for treating patients without delay, unlike existing autologous cell therapies.” The goal of Nkarta’s platform is to maximize the therapeutic effects of allogeneic NK cells through robust expansion, enhanced targeting and extended persistence to obtain a powerful and sustained anti-cancer immune-mediated attack. The combination of its cell expansion and cryopreservation platform with CRISPR-based genome engineering technologies, Nkarta is building an interesting pipeline of cancer therapies.
In June 2023, Nkarta presented preliminary data based on a November 2022 data cut-off from its Phase 1 dose escalation clinical trial of NKX019 (CAR NK product targeting CD19).
What are NK cells?
Natural killers, or NK cells, are cytotoxic innate lymphoid cells. They consist in the body’s first line of defense against viral infections and cancer cells with an innate ability to rapidly seek and destroy transformed cells. NK recognize and kill cells lacking major histocompatibility complex (MHC) class I expression. According to Nkarta, “NK cell therapy has the potential to 1) target multiple pathogenic antigens with measurably more efficient cytotoxicity, 2) be better controlled to reduce risk of cytokine storms and 3) be produced from a variety of sources without relying on patient-specific immune cells”.
Details of the NKX019 clinical trial
The study was first posted in August 2021 and is still recruiting (NCT05020678). This is a single arm, open-label, multi-center, Phase 1 study to determine the safety and tolerability of the experimental therapy NKX019 (allogeneic CAR NK cells targeting CD19) in patients with relapsed/refractory non-Hodgkin lymphoma, chronic lymphocytic leukemia, or B cell acute lymphoblastic leukemia.
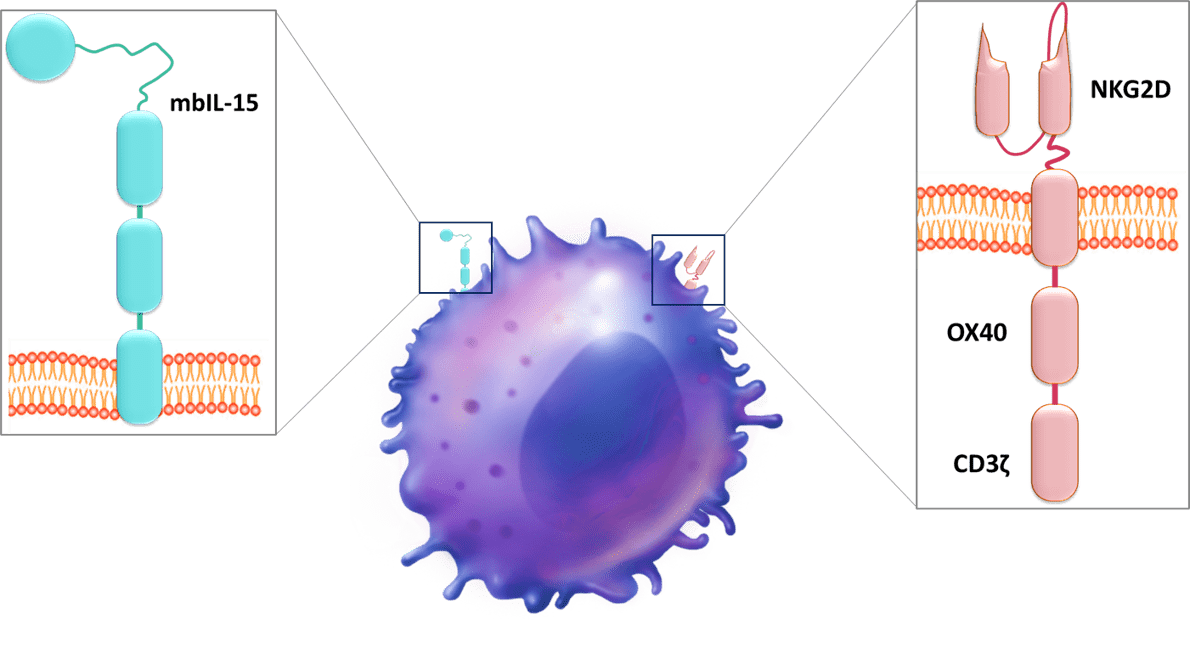
Fig.1: Nkarta NKX019 (a CAR NK product that targets CD19)
This trial is conducted in 2 parts. The part 1 is a dose finding utilizing a “3+3” enrollment schema and safety lead-in to confirm dose for NKX019 in combination with rituximab (monoclonal antibody anti-CD20) expansion cohorts (as applicable). The starting dose is 3 × 108 NK cells (6 × 106/kg for patients < 50 kg) administered as 3 weekly doses. The part 2 is a dose expansion to further evaluate safety and tolerability, cellular kinetics, pharmacodynamics, and anti-tumor response in expansion cohorts of patients.
Results from the initial data revealed a complete response in seven out of ten patients with relapsed/refractory non-Hodgkin lymphoma that had been treated with higher doses of CAR NK cells (1 billion and 1.5 billion per dose). This included two patients with aggressive large B cell lymphoma and other difficult histologies, such as mantle cell lymphoma, high-risk follicular lymphoma, and marginal zone lymphoma.
In the study, there was no dose-limiting toxicity, ICANS, GVHD, or Grade 3 cytokine release syndrome observed. David R. Shook, Nkarta’s Chief Medical Officer stated that “these data show a hopeful safety profile and clinical effectiveness across different histologies in the dose escalation part of the NKX019 study.”
Nkarta’s patent families related to NKX019 clinical trial
The company has 14 patent families and two of them describe a CD19 CAR. First, Nkarta filed in 2020 a patent family which details a CD19 CAR construct (WO2020/180882). This patent family, claiming immune cell that expresses a CD19-directed CAR, a polynucleotide encoding a CD19-directed CAR and a method of treating a cancer using CD19-directed CAR, comprises several US-granted patents (US11154575, US11253547, US11141436) and patent applications in EP, JP, CN and other foreign countries. In the technology described in this document, the CAR, encoded by a polynucleotide, comprises an extracellular anti-CD19 binding moiety (wherein the anti-CD19 binding moiety comprises a variable heavy (VH) domain of a single chain fragment variable (scFv) and a variable light (VL) domain of a scFv), a CD8 alpha hinge, and a transmembrane domain. The transmembrane domain comprises a CD8 alpha transmembrane domain and an intracellular signaling domain (0X40 subdomain and a CD3 zeta subdomain). Moreover, membrane-bound interleukin-15 (mbIL-15) is expressed on the CAR cell. mbIL-15 can be encoded on the same construct or on a separate construct. The IL-15 domain, e.g., mbIL-15 domain, may act as a co-stimulatory domain. It supports prolonged cell survival and proliferation and may render immune cells expressing it particularly efficacious against target tumor cells. IL-15 enables for greater persistence and cell activity without exogenous cytokine support. To develop this allogeneic CAR, NK cells are isolated from peripheral blood mononuclear cells and expanded through the use of a feeder cell line. Then, CAR NK cells were generated by transduction with viruses encoding CD19 CAR, with the goal of treating a variety of B cell malignancies. Data demonstrated that CD19-directed CAR constructs can not only be expressed, but are stably expressed, and are also effective in inducing cytotoxicity in multiple cancer cell types, in some cases exceeding an 80% kill rate.
Then, Nkarta filed another patent family also in 2020, which relates to a population of genetically engineered natural killer cells for cancer immunotherapy (WO2020/247392). This patent family claiming genetically engineered NK cells as described below comprises patent applications in the US, EP, JP and other foreign countries. This document describes NK cells engineered to express a cytotoxic receptor, with an extracellular ligand binding domain comprising an anti-CD19 antibody fragment, a CD8 transmembrane domain, and a cytotoxic signaling complex (OX40 subdomain and a CD3zeta subdomain). NK cells also express membrane-bound IL-15 (mblL15). Moreover, NK cells are genetically edited to express reduced levels of a cytokine-inducible SH2-containing (CIS) protein encoded by the CISH gene. When CISH is knocked out, IFNγ production is notably increased, and its combination with CAR19 expression results in nearly 2.5 times more IFNγ production than the CISH knockout alone. Similar data is seen with TNFα production. The doubly modified cells exhibit a more robust (e.g., cytotoxicity-inducing) cytokine profile and/or increased viability/persistence, which allows for a greater overall anti-tumor effect. To engineer NK cells, cryopreserved, purified NK cells were thawed on Day 0 and subject to electroporation with CRISPR/Cas9 and a single (or two) CISH guide RNAs. On Day 7, knockout efficiency was determined, and the NK cells were transduced with a virus encoding the CD19 NK CAR construct. On Day 14, knockout efficiency was determined, and cytotoxicity of the resultant NK cells was evaluated. Experiments showed that NK cells were edited (knockout CISH expression) to enhance one or more NK cell characteristics through IL15-mediated signaling, in addition to expressing the anti-tumor CAR. This engineering and editing combination yields synergistic enhancements to NK cell function, such as expansion, cytotoxicity, and/or persistence.
To conclude, although NK cells still remain little used in allogeneic CAR area, it is a very promising technology that Nkarta has completely taken over. In addition, the first results in its clinical trial are encouraging and its bold strategy may be rewarded.
Discover KnowMade‘s IP expertise in the field of medtech.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About the author
Fabienne Massa works at KnowMade in the field of Biotechnology and Life Sciences. She holds a PhD in Molecular and Cellular Biology from the IPMC (Nice, France). She also holds a Master of Business Management from IAE (Nice, France) and she previously worked in the pharmaceutical industry.
About KnowMade
KnowMade is a technology intelligence and IP strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patent information and scientific literature into actionable insights, providing high added value reports for decision makers working in R&D, innovation strategy, intellectual property, and marketing. Our experts provide prior art search, patent landscape analysis, freedom-to-operate analysis, IP due diligence, and monitoring services.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Sensors, Semiconductor Packaging, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
