SOPHIA ANTIPOLIS, France – January 16, 2026 │ This quarter, KnowMade’s Therapeutic mRNA patent monitoring service highlights PopVax as an emerging innovator driving multivalent mRNA vaccines and next-generation delivery platforms.

Identifying Emerging mRNA Innovators Through Patent Surveillance: The Case of PopVax
Patent Monitoring as a Strategic Lens on Therapeutic mRNA Innovation
Patent monitoring has become a critical tool for tracking innovation dynamics in therapeutic mRNA, a field characterized by rapid technological iteration, dense intellectual property landscapes, and increasing geographic diversification. Dedicated patent analytics services—such as the Therapeutic mRNA Patent Monitor—are now widely used to identify emerging technology trends, platform shifts, and rising innovators well before these developments become visible through clinical pipelines or commercial partnerships. Insights from therapeutic mRNA patent monitoring in 2023 and 2024 revealed a sustained high level of innovation, with more than 600 new patent families published in 2023 and a further increase to over 730 in 2024, reflecting continued competitive pressure across the ecosystem. At the same time, patent monitoring underscored the rising prominence of Asian players, particularly Chinese companies such as Abogen and Anovent in delivery technologies, alongside increasing activity from India—setting the stage for the emergence of new platform-oriented innovators in the global therapeutic mRNA patent landscape.
Beyond established leaders in North America and Europe, systematic patent surveillance has proven particularly valuable in highlighting new sources of innovation in emerging countries, where mRNA capabilities are expanding rapidly and often outside traditional venture-backed ecosystems. In this context, patent activity functions not only as a proxy for R&D intensity, but also as an early indicator of strategic ambition and platform maturity.
PopVax as an Emerging Signal in Global mRNA Patent Activity
Within this landscape, PopVax represents a noteworthy case. While the company is not a strict newcomer to the mRNA field, its patenting activity shows a clear acceleration and qualitative shift beginning in Q4 2025, marking a transition from exploratory filings toward a more structured and platform-driven intellectual property strategy. This trajectory mirrors patterns previously observed in other emerging mRNA players that were early identified through patent monitoring—most notably Chinese companies such as Abogen, whose rapid portfolio expansion preceded broader international visibility.
PopVax’s geographic origin further reinforces its relevance from a monitoring perspective. As an India-based company, PopVax reflects the country’s growing ambition to position itself as a significant contributor to next-generation mRNA therapeutics and vaccines, leveraging strengths in biologics manufacturing, cost efficiency, and public-health–oriented innovation. The recent expansion of its patent portfolio—spanning delivery technologies, mRNA constructs, and multivalent vaccine platforms—suggests that India may increasingly feature alongside China as a key emerging geography in global therapeutic mRNA patent landscapes.
PopVax: Company Overview and Strategic Positioning
Building End-to-End mRNA Capabilities in India
PopVax is a privately held Indian biotechnology company founded in 2021 and initially incubated at the Atal Incubation Centre – Centre for Cellular & Molecular Biology (AIC-CCMB). Its core scientific and operational activities are based in Hyderabad, Telangana, where the company has established an integrated “RNA Foundry” combining discovery research with GMP-capable clinical manufacturing infrastructure.
The company’s mission is to build a full-stack RNA biopharmaceutical platform in India, encompassing antigen discovery, mRNA construct design, delivery technologies, and clinical manufacturing. Central to this strategy is the integration of machine-learning–driven antigen design with experimental immunology and RNA engineering. PopVax applies computational and machine-learning models to explore large antigen sequence spaces, identify conserved and immunologically relevant regions, and design optimized vaccine immunogens with the potential for broad and durable immune responses. These in silico predictions are iteratively refined through experimental validation, creating a feedback loop that accelerates antigen optimization and candidate selection.
Pipeline Priorities and Technology Differentiation
PopVax’s development pipeline focuses primarily on next-generation mRNA vaccines, including broadly protective candidates for COVID-19 and influenza, alongside earlier-stage programs targeting additional infectious diseases, as described in the pipeline illustrated in figure 1, and select oncology indications. A key differentiating axis is the company’s emphasis on thermostable mRNA formulations, designed to reduce cold-chain dependency and improve vaccine accessibility in low- and middle-income settings.
| Program | Deaths per year caused by pathogen | Status of program | Phase I trial timeline |
| Broadly-protective influenza | ≈ 400K | Late preclinical | 2026 |
| HCV | ≈ 240K | Late preclinical | 2027 |
| Prophylactic + Therapeutic HPV | ≈ 350K | Design Phase | 2028 |
| Strep A | ≈ 500K | Design Phase | 2028 |
| Tuberculosis | ≈ 1M | Design Phase | 2029 |
Figure 1: PopVax current mRNA vaccines related pipeline.
From PopVax Website
From a funding and partnership perspective, PopVax relies predominantly on non-dilutive public-health and philanthropic funding rather than traditional venture capital. Key supporters include the Bill & Melinda Gates Foundation (1.15 Million USD for thermostable mRNA delivery), BARDA (U.S.) (2 Million USD as One of the Winners of the BARDA Patch Forward Prize for its Seasonal Influenza Vaccine), and global biosecurity initiatives such as Balvi. On the clinical side, NIAID/NIH is conducting and sponsoring a U.S.-based Phase I clinical trial of PopVax’s next-generation mRNA-LNP COVID-19 vaccine under the U.S. Government’s Project NextGen, providing external validation of the platform’s translational potential.
PopVax Announces Potent, Broadly Protective mRNA-Based Influenza Vaccine Candidates
In early 2025, PopVax shared an update on progress toward broadly-protective influenza vaccines designed to provide immunity not only against seasonal flu strains but also against potential pandemic variants such as H5N1. According to the announcement, PopVax’s influenza vaccine designs elicit significantly higher antibody responses in preclinical models compared with existing licensed seasonal vaccines—up to 250-fold greater antibody titers in mice against key seasonal strains and robust responses against H5N1, despite the vaccine not encoding an H5N1-specific immunogen, these results are illustrated in figure 2.
These broadly-protective candidates leverage PopVax’s machine learning–enabled immunogen design and mRNA architecture technologies, which aim to generate immune responses that extend beyond strain-specific protection.
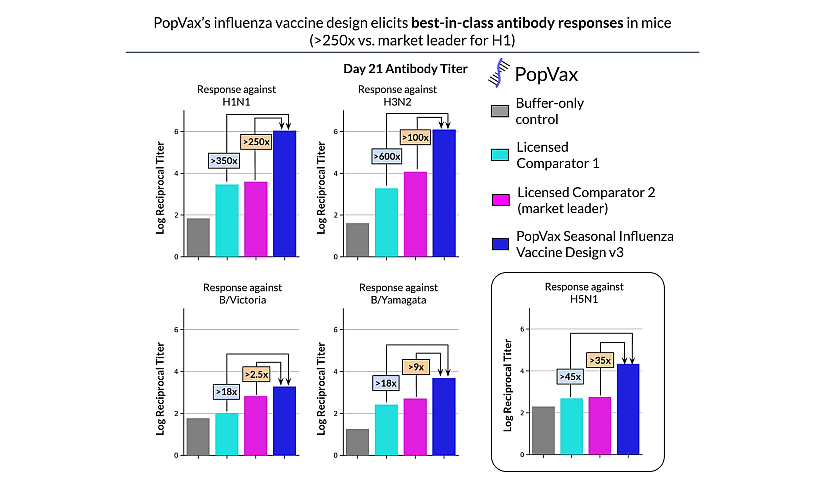
Figure 2: PopVax Influenza Vaccine efficiency.
From PopVax Website
From Delivery to Multivalent Vaccines: An IP-Driven Analysis
Establishing the Core: LNP Systems and Antigen Constructs
As of the most recent update, PopVax’s intellectual property portfolio comprises 13 patent families related to therapeutic mRNA technologies, reflecting a deliberately balanced strategy. Of these, six patent families focus on lipid nanoparticle (LNP) delivery systems, while seven patent families cover mRNA constructs and vaccine antigens, underscoring a dual emphasis on payload engineering and delivery optimization.
The company’s earliest filings in 2023, establish this technological foundation. One patent family (WO2024/044178) focuses on LNP compositions comprising nucleic acids, designed to improve stability, encapsulation efficiency, and intracellular delivery of DNA and RNA payloads. Two additional families address therapeutic mRNA design, including constructs encoding betacoronavirus-derived antigens such as receptor-binding domains, fusion peptides, and stem helices (WO2024/141784), as well as multisequence nucleic acid architectures enabling structurally optimized or multivalent mRNA vaccines (WO2024/141786).
Portfolio Acceleration and Platform Expansion (2026)
A major inflection point occurs in 2026, with two successive waves of patent filings that significantly expand both the depth and breadth of the portfolio. In April 2026, PopVax filed five closely related patent families (WO2025/217121, WO2025/217125, WO2025/217129, WO2025/217131, and WO2025/217133) dedicated to novel LNP delivery systems. These families collectively establish a broad chemical and formulation platform based on newly designed cationic lipids, enabling fine control over physicochemical properties such as charge behavior, biodegradability, and membrane interaction. The claims emphasize versatility, covering delivery of mRNA as well as other nucleic acid modalities across therapeutic and prophylactic applications.
This delivery-focused expansion is followed, in June 2026, by the filing of five additional patent families (WO2026/003577, WO2026/003585, WO2026/003578, WO2026/003579, and WO2026/003586) targeting multivalent mRNA vaccines against major viral pathogens, including hepaciviruses, influenza, pediatric respiratory viruses (RSV, HMPV, HPIV), papillomaviruses, and herpesviruses. These families are built around a shared multisubunit nucleic acid architecture, in which a single long-form mRNA encodes multiple antigens or epitopes in a modular format.
From an IP perspective, these filings extend protection across multisubunit mRNA constructs, expressed polypeptides and nanoparticle assemblies, vaccine compositions, and methods of use, while preserving manufacturing simplicity through single in vitro transcription–based production. Collectively, they signal a shift from component-level innovation toward a fully platformized, pathogen-agnostic vaccine architecture.
Strategic Takeaways from PopVax’s Patent Trajectory
PopVax’s patent trajectory illustrates how systematic patent monitoring enables early identification of strategic inflection points in emerging therapeutic mRNA innovators. The company’s transition from foundational delivery and antigen-focused filings toward platform-scale intellectual property, spanning lipid nanoparticle chemistry, multisubunit mRNA constructs, and multivalent vaccine architectures, positions PopVax as a credible long-term platform player rather than a single-program developer. More broadly, its evolution reinforces the view that India is emerging as a meaningful geography in the global therapeutic mRNA ecosystem, alongside China, with growing implications for competition, partnerships, and technology diffusion.
From an IP analytics perspective, this case study underscores the value of continuous therapeutic mRNA patent surveillance in capturing not only incremental innovation, but also structural shifts in technology strategy, portfolio maturity, and geographic innovation dynamics. By tracking patent activity at a granular level, patent monitoring services can reveal how next-generation RNA platforms, delivery technologies, and multivalent vaccine concepts are shaping the future of therapeutics and biotechnology well ahead of clinical or commercial visibility.
KnowMade’s patent monitoring services continue to capture and contextualize these pivotal developments, providing decision-makers with actionable insights into emerging mRNA technologies, competitive positioning, and innovation trajectories across global markets. For organizations seeking to understand how advances in therapeutic mRNA and RNA delivery may impact their R&D strategy, IP positioning, or business development priorities, KnowMade offers in-depth analyses and tailored monitoring solutions—please contact us to learn more.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About the author
Elodie Bovier, PhD., works at KnowMade as a Patent Analyst in the field of Biotechnology and Life Sciences. She holds a PhD in genetic and molecular biology from the Paris Sud University. She also holds the Industrial Property International Studies Diploma (in Patent and Trademark & Design Law) from the CEIPI (Strasbourg, France).
About KnowMade
KnowMade is a technology intelligence and IP strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patent information and scientific literature into actionable insights, providing high added value reports for decision makers working in R&D, innovation strategy, intellectual property, and marketing. Our experts provide prior art search, patent landscape analysis, freedom-to-operate analysis, IP due diligence, and monitoring services.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Sensors, Semiconductor Packaging, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.cahue
