SOPHIA ANTIPOLIS, France – July 16, 2025 │ KnowMade actively tracks therapeutic mRNA innovations as part of its Therapeutic mRNA patent monitoring service. What’s new today?
Spotlight on Emerging Innovators: The Strategic Value of Patent Monitoring
The first half of 2025 confirms the ongoing momentum in therapeutic mRNA innovation, as highlighted in KnowMade’s press release for Q2 2025. A total of 272 new patent publications were identified in Q2, following the 257 recorded in Q1, demonstrating a consistent upward trend in intellectual property activity across the field.
One of the key strengths of KnowMade’s patent monitoring service lies in its ability to detect emerging players at the forefront of technological innovation. Through systematic tracking of first-time patent filings, the service provides early visibility into startups and academic spin-offs often before they secure major funding or public recognition. Notable examples include the identification of AGS Therapeutics in 2023, a French biotech innovating with microalgae extracellular vesicles (MEVs) for mRNA delivery, see our detailed Insight from November 2024, as well as Pinion Immunotherapeutics, Arcalis, and Amplitude Therapeutics in 2025.
Cila therapeutics, identified this quarter, is a company not focused on mRNA technology, but that develops an interesting delivery system targeting the lung.
More Than RNA: CILA’s Dual Strategy in Respiratory Therapeutics
A Pipeline Built for Pulmonary Precision
Founded in 2018 and headquartered in Boston, Massachusetts, CILA Therapeutics is a privately held biotech company focused on developing inhaled therapies for both common and rare respiratory conditions. Led by Dr. Safia K. Rizvi, a seasoned biopharma executive, the company is advancing treatments for diseases such as Primary Ciliary Dyskinesia (PCD), COPD, severe asthma, bronchiectasis, cystic fibrosis, and viral infections. For treating patients affected by such pathologies, a silent enemy reduces the effectiveness of inhaled therapies: the mucus (see figure 1). Normal mucus protects the respiratory apparatus by trapping undesired elements which is cleared by the mucociliary elevator. However, genetic and environmental factors cause mucus overproduction and/or diminished mucociliary clearance in obstructive airway diseases. This leads to mucus build-up and the formation of “Pathological Mucus Plugs” – a mesh-like network of mucin proteins that form thick tenacious mucus plugs that are difficult to clear.
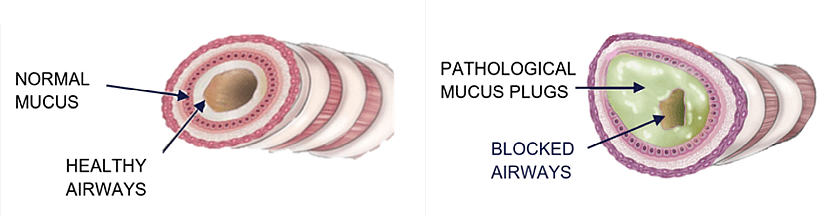
Figure 1: Illustration of normal and obstructive airways. From CILA website.
The company’s flagship product, CIL-05, is a non-RNA-based therapy designed to reduce mucus viscosity and enhance airway clearance in PCD patients. It uses low-density respirable microspheres encapsulating stabilized mucolytic agents like sodium 2-mercaptoethane sulfonate and DNase, delivered via a dry-powder inhaler (DPI). Currently in preclinical development, CIL-05 is expected to enter clinical trials within the next 12 months following pre-IND meetings with the FDA (see figure 2).
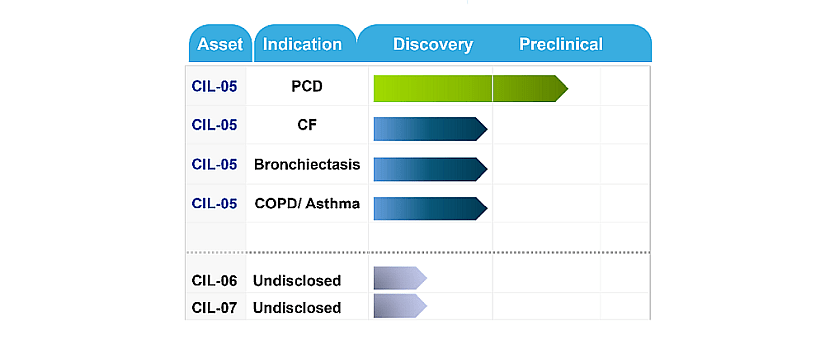
Figure 2: Current CILA pipeline. From CILA website.
Beyond CIL-05, CILA is developing a co-therapy platform focused on nucleic acid delivery, particularly RNA and DNA, to lung and airway cells. The company holds an international patent application supporting this work, WO2025101704, filed on 2024-11-26.
A Lipid-Based Delivery System Targeting RNA Therapeutics to the Lungs
CILA’s international patent application WO2025101704 outlines a proprietary lipid-based system optimized for the targeted delivery of RNA molecules to pulmonary tissues. The invention addresses long-standing limitations in lung-directed RNA therapy, such as degradation in the extracellular environment and inefficient uptake by target cells. By encapsulating RNA within lipid vesicles, such as liposomes or lipid nanoparticles, the platform ensures that therapeutic RNAs remain stable, bioavailable, and effectively internalized by lung epithelial cells.
Key innovations lie in the compositional tuning of lipid mixtures, including cationic lipids, phospholipids, and cholesterol derivatives that promote efficient RNA encapsulation and endosomal escape. Indeed, the claimed invention is a composition for enhanced delivery of nucleic acids to lung and airway cells, characterized in that it includes at least a transfection efficiency enhancing agent and a therapeutic nucleic acid with a delivery vehicle. The delivery system is engineered to operate under aerosolized conditions suitable for inhalation therapies, aligning with CILA’s broader inhaled treatment strategy.
According to inventors, the invention offers significant improvements over prior art by enhancing transfection efficiency of nucleic acids, thereby increasing therapeutic efficacy while minimizing systemic exposure and potential side effects. This is supported by preclinical experiments cited in the patent application that demonstrate successful delivery and functional expression of RNA cargos in lung tissues following administration (see figure 3). Nevertheless, it is important to note that these findings are based exclusively on in vitro cellular models. As such, the translational relevance to in vivo contexts remains to be established, and further preclinical studies in animal models are necessary to confirm the efficacy and safety of the approach within the complex environment of a living organism.
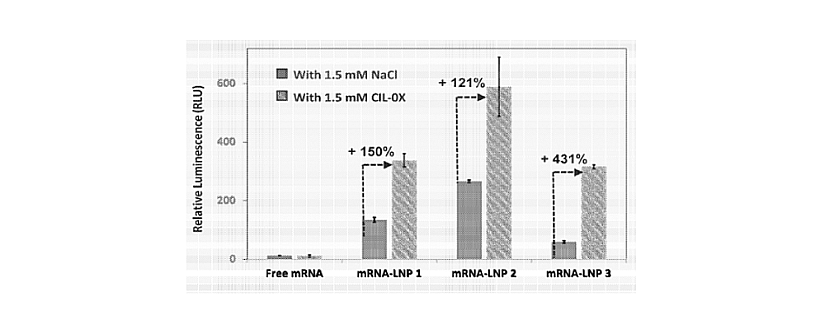
Figure 3: CIL-0X enhancing effect.
The graph illustrates the percentage increase in transfection of nucleic acid in primary human bronchial epithelial cells with and without prior exposure to transfection-enhancing agent CIL-0X. Luciferase mRNA was prepared in 4 different forms, including free mRNA without a delivery vehicle and in three different lipid nanoparticle vehicles, i.e., mRNA-LNP-1, mRNA LNP-2, mRNA-LNP3. The expression of Luciferase was assessed by measuring Luciferase enzyme activity, after 24 hours of nebulized administration of free mRNA and mRNA in LNPs vehicles to the primary human epithelial cells that were pre-treated with either 1.5 mM CIL-OX (transfection enhancing agent/composition) as the TEST arm or 1.5 mM NaCl as the CONTROL arm, 30 minutes before mRNA-LNP administration.
From WO2025101704.
This innovation addresses the challenges of delivering therapeutic nucleic acids effectively to the respiratory system, particularly in patients with conditions like cystic fibrosis and primary ciliary dyskinesia. By utilizing specific agents that enhance transfection efficiency, the invention significantly improves the uptake of therapeutic nucleic acids, thereby increasing their effectiveness. This targeted approach not only enhances the therapeutic potential of nucleic acid-based treatments but also reduces the risk of systemic side effects, making it a promising solution for a wide range of pulmonary diseases.
CILA’s Hybrid Strategy, a plus in a specific landscape
Despite the breakthroughs in systemic mRNA delivery seen during the COVID-19 pandemic, targeted delivery to the lungs remains a major challenge, and opportunity, within RNA therapeutics. The lung’s complex architecture and protective mucosal barriers complicate the effective delivery of nucleic acids, particularly in chronic respiratory conditions. Established players like Translate Bio (acquired by Sanofi) and Arcturus Therapeutics have long explored inhaled mRNA delivery, particularly for cystic fibrosis. More recently, emerging companies such as ReCode Therapeutics have entered the field, each developing proprietary LNPs and delivery vectors optimized for airway administration. Still, most delivery innovations remain systemically focused, leaving a gap for specialized approaches tailored to pulmonary indications.
Among the respiratory diseases that could benefit from localized RNA therapeutics, Primary Ciliary Dyskinesia (PCD) stands out due to its genetic etiology and localized pathology. Characterized by impaired mucociliary clearance and chronic respiratory infections, PCD lacks targeted treatments beyond symptomatic management. CILA Therapeutics is addressing this gap through CIL-05, an inhaled dry-powder mucolytic designed to enhance airway clearance in PCD patients. While CIL-05 is a non-RNA candidate, it reflects CILA’s deep investment in pulmonary medicine. In parallel, the company is exploring delivery enhancements that could benefit broader applications—including cystic fibrosis, pulmonary fibrosis, and rare monogenic lung disorders—where inhaled RNA therapeutics could offer transformative outcomes.
CILA’s approach integrates near-term clinical development with long-term platform innovation. This hybrid model allows the company to de-risk its business by advancing a marketable therapeutic (CIL-05) while concurrently building proprietary delivery technologies that enhance nucleic acid uptake in lung cells. By focusing on disease-relevant delivery, rather than generalized transfection, CILA distinguishes itself from larger players and positions its platform for both in-house programs and future partnering opportunities in respiratory gene therapy.
Conclusion
Innovation in therapeutic mRNA continues to accelerate, and Q2 2025 highlights how impactful contributions increasingly come not only from established leaders, but also from specialized newcomers. CILA Therapeutics exemplifies this trend through its dual focus on mucolytic inhaled treatments and RNA-enhancing delivery systems for respiratory applications. Although its lead asset, CIL-05, is not RNA-based, the company’s patent activity—particularly WO2025101704—demonstrates a clear strategic commitment to overcoming one of mRNA’s greatest challenges: effective pulmonary delivery.
This hybrid model—combining near-term clinical programs with long-term platform innovation—mirrors the adaptability that the RNA therapeutic field demands. Alongside Amplitude Therapeutics, which is advancing trans-amplifying RNA technologies, CILA is reshaping the delivery landscape with application-specific, scalable solutions. These developments underscore a broader shift toward precision delivery technologies tailored to complex indications like cystic fibrosis and PCD.
Crucially, KnowMade’s patent monitoring service enables early identification of such innovators by analyzing first-time patent filings. In a field as dynamic as mRNA therapeutics, timely and focused IP intelligence is key to staying ahead of the curve. KnowMade remains committed to delivering these strategic insights across the global innovation ecosystem. For more detailed insights into these developments and how they may impact your business, please contact us.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About the author
Elodie Bovier, PhD., works at KnowMade as a Patent Analyst in the field of Biotechnology and Life Sciences. She holds a PhD in genetic and molecular biology from the Paris Sud University. She also holds the Industrial Property International Studies Diploma (in Patent and Trademark & Design Law) from the CEIPI (Strasbourg, France).
About KnowMade
KnowMade is a technology intelligence and IP strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patent information and scientific literature into actionable insights, providing high added value reports for decision makers working in R&D, innovation strategy, intellectual property, and marketing. Our experts provide prior art search, patent landscape analysis, freedom-to-operate analysis, IP due diligence, and monitoring services.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Sensors, Semiconductor Packaging, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.cahue
