SOPHIA ANTIPOLIS, France – August 31, 2020 | Biofidelity recently announced that it has raised $12 million, completing its Series A financing. The company disclosed that the series A investors are BlueYard Capital, Longwall Ventures and Agilent Technologies. Biofidelity is, above all, focused on developing non-small cell lung cancer detection assays that surpass the performance of PCR-based assays. Biofidelity’s technology could later be adapted to cover several applications including the following:
• Resistance monitoring
• Recurrence monitoring
• Minimal Residual Disease (MRD) monitoring
• Screening for early cancer detection
• Organ transplant rejection early detection
• Non-invasive prenatal testing
Biofidelity‘s patent application (WO2020/016590 Improved polynucleotide sequence detection method) has been identified by Knowmade in its Cancer Diagnostics Startup Identification report. Since patent applications must be examined and published by the patent offices, they are a useful source of information on companies that do not provide many details about the technology they are developing, such as Biofidelity. The invention claimed in the WO2020/016590 application aims at providing a new method for selectively amplifying nucleic acid sequences that would overcome some of the drawbacks of the classical polymerase chain reaction (PCR). When multiple nucleic acid sequences are present in a sample, PCR is prone to generating false positives resulting from unspecific amplification. In PCR-based methods, the quantification of the target analyte remains imprecise. Furthermore, PCR is sensitive to mutations in the targeted sequence. False negatives can occur when the genetic region targeted by the test primers underwent a mutation. The detection of a specific single nucleotide polymorphism (SNP) can lead to false positives when the wild-type variant is also present in the sample. Biofidelity is confident that its technology will overcome these drawbacks by involving the double-strand specificity of pyrophosphorolysis in its polynucleotide sequence detection method.
The method for which Biofidelity is seeking a patent comprises 5 steps that can be summarised as follows:
1) Annealing the analyte (i.e. a single-stranded nucleic acid) to a single-stranded probe oligonucleotide A0 to create a first intermediate product that is at least partially double-stranded and in which the 3′ end of A0 forms a double-stranded complex with the analyte target sequence;
2) Performing the pyrophosphorolysis of the first intermediate product with a pyrophosphorolysing enzyme in the 3′-5′ direction from the 3′ end of A0 to create a partially digested strand A1 and the analyte;
3) Creating an oligonucleotide A2, derived from the sequence of A1 (e.g. by annealing A1 with a probe B, by circularising A1, or by ligating the extremities of A1);
4) Amplifying the oligonucleotide A2; and,
5) Detecting a signal derived from the multiple copies of A2 (e.g. by performing a melting curve analysis) and inferring therefrom the presence or absence of the polynucleotide target sequence (e.g. a gene, an allele, a mutation) in the analyte.
Even if the claimed embodiments of the invention work with a pyrophosphorolysis enzyme, the inventors disclosed that the invention could also work with a double-strand specific exonuclease (e.g. a 3′-5′ exonuclease such as Exolll or a 5′-3′ exonuclease such as Lambda Exo), instead of the pyrophosphorolysis enzyme.
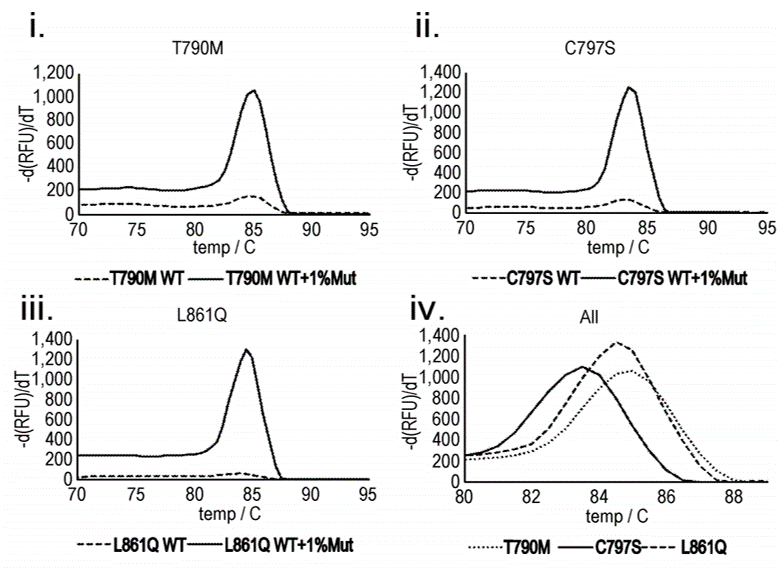
For example, epidermal growth factor receptor (EGFR) inhibitors, such as gefitinib and erlotinib, are commonly used as first-line treatments for non-small cell lung cancer (NSCLC). During treatment, the tumour will often develop mutations in the EGFR gene (e.g. T790M, C797S) that confers resistance to the treatment. Early detection of these mutations means the patient can be switched to alternative therapies.
The figure shows the melt peak results for amplification products produced from a rolling circle amplification using primers for three different mutations that can occur to the EGFR gene: (i)T790M (exon 20), (ii) C797S (exon 20) and (iii) L861Q (exon 21). The temperature was raised to 95°C, with measurements taken at 0.5°C intervals. In (iv), the position of the melting peak can be used to identify which mutation i.e. T790M, C797S or L861Q is present. It can be seen that the presence of a significant melting peak can thus be used to infer the presence of the mutation targeted by a given probe, while the position of this peak can be used to identify the nature of the mutation.
The European patent application EP3682030 (resulting from the PCT application WO2020/016590) is currently under examination. Biofidelity amended the claims of its patent application to explicitly mention some essential features of the invention to try to overcome the objections of the European Patent Office (EPO) examiner.
Some of the inventors of the above-mentioned application (Dr Barnaby Balmforth, Cameron Frayling, and Dr Ana Silva-Weatherley) are also working on two other start-up projects: Base4 Innovation (a company developing a new sequencing technology) and Lightcast Discovery (a company developing a microdroplet manipulation system). The three companies are domiciled at the same address: the Broers Building, on the West Cambridge Campus of the Cambridge University.
The company announced, in January 2020, the successful completion of its study to detect specific mutations in lung cancer using a test developed in-house. This study was carried out in collaboration with Agilent Technologies and supported by InnovateUK (under grant No. 105202) as part of the Investment Accelerator: Innovation in Precision Medicine program. According to Biofidelity, the results obtained show an increase in the sensitivity of approximately 50 times compared to the PCR-based tests currently approved by the FDA. The performance achieved with Biofidelity’s technology matches that of specialised Next Generation Sequencing (NGS) assays, while requiring a simpler workflow and standard laboratory instrumentation. The company also claims that its assay achieved perfect specificity as no false positives were observed in the detection of multiplexed panels of mutations from both tissue and plasma, on more than 750 assays. Given the results of this study, finding Agilent Technologies among Biofidelity’s Series A investors is no surprise.
Since very little information about Biofidelity’s technology is available from its website and because of the close relationship the company has cultivated with Agilent Technologies, it seems highly likely that the latter will acquire the license for the assays developed by the Biofidelity, or even acquire the company.
All our Healthcare patent reports.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About our analysts
Olivier THOMAS, MSc. Olivier works at Knowmade in the field of Biotechnology and Life Sciences. He holds a MSc in Molecular and Cellular Biology from the UPMC (Paris, France). He also holds the Industrial Property International Studies Diploma (Patent, Trademark and Design Law) from the CEIPI (Strasbourg, France).
About Knowmade
Knowmade is a Technology Intelligence and IP Strategy consulting company specialized in analysis of patents and scientific information. The company helps innovative companies and R&D organizations to understand their competitive landscape, follow technology trends, and find out opportunities and threats in terms of technology and patents.
Knowmade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patents and scientific information into business-oriented report for decision makers working in R&D, Innovation Strategy, Intellectual Property, and Marketing. Our experts provide prior art search, patent landscape analysis, scientific literature analysis, patent valuation, IP due diligence and freedom-to-operate analysis. In parallel the company proposes litigation/licensing support, technology scouting and IP/technology watch service.
Knowmade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Solid-State Sensors/Actuators, Semiconductor Manufacturing, Packaging & Assembly, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
