SOPHIA ANTIPOLIS, France – August 24, 2020 | The race to find a COVID-19 vaccine has highlighted several microneedle technologies for their advantages over common parenteral injections. Microneedles have shown immense potential, since they can efficiently, painlessly and safely deliver a vaccine through the skin. Microneedles reach the underlying dermis and epidermis, rich in antigen presenting and accessory cells of the immune system, promoting potent and durable adaptive immunity, thereby reducing the dose of vaccine required. Furthermore, microneedle-based vaccines can be stored outside of the cold chain and be self-administered by anybody, thereby eliminating the need for skilled medical practitioners. This serves as an important advantage, in order to reach all of the population in need, especially in developing countries. Driven by the urgent need for a COVID-19 vaccine, microneedles could be a game-changer and replace standard parenteral injections.
In this race, in June 2020, Merck acquired Themis Bioscience (‘Themis’), an Austrian company that licensed a vaccine platform developed at the Pasteur Institute, based on the established measles vaccine. Themis has been part of a consortium, since March 2020, including the Pasteur Institute and the Center for Vaccine Research at the University of Pittsburgh, to develop a vaccine candidate targeting SARS-CoV-2 to prevent COVID-19.
Promising results were released in June 2020 from the University of Pittsburgh on the application of their dissolvable microneedle PittCoVacc vaccine in mice, showing the production of antibodies specific to the SARS-CoV-2 spike gene, in quantities thought to be enough to neutralise the virus. IND approval was requested from the FDA, and a Phase I human clinical trial should start in the next few months. Based on the KnowMade’s report Microneedles for drug delivery patent landscape published in August 2020, the patent WO2013166162 filed by the University of Pittsburgh and Carnegie Mellon University was identified. This patent family discloses a tip-loaded microneedles array wherein the active compounds are incorporated into the needle tips thereby improving the efficiency of the delivery and reducing the waste of the non-deliverable active components incorporated into the non-needle portions of the array (Figures A and B, below). This broad patent family comprises numerous pending applications and granted patents in the US, Europe, Japan, China, Canada, etc.

Figures A and B illustrate the tip-loaded microneedle arrays wherein the active component is concentrated in the microneedle tips. The microneedle arrays are fully dissolvable and have unique microneedle geometries that enable the effective delivery of a broad range of active components, including vaccines (see WO2013166162).
In May 2020, Merck also exercised its option to use the Vaxxas High-Density Microarray Patch (HD-MAP) platform as a vaccine candidate, but the vaccine candidate was not disclosed. However, Merck could use the dime-sized Vaxxas patch to deliver its new vaccine. Vaxxas HD-MAP is a 9 x 9 mm array with thousands of very short (~ 250 µm) projections, which is notably disclosed in patent application WO201754040, claiming an array with a density of at least 2,000 microneedles per cm and an applicator to control velocity during the application. This technology improves the immunogenicity of vaccine preparations by efficiently delivering the vaccine at the appropriate depth. According to KnowMade’s report Microneedles for drug delivery patent landscape, this patent family is currently pending in the US, Canada, the European Union and Australia.
Other companies, such as Kindeva (formerly 3M Drug Delivery Systems, launched as an independent company in May 2020), Debiotech (DebioJectTM Microneedle), BioSerenTach (percutaneous dissolving microneedles) and Nanopass (MicronJetTM microneedle) are collaborating with vaccine companies by providing their microneedle vaccine delivery platform technologies.
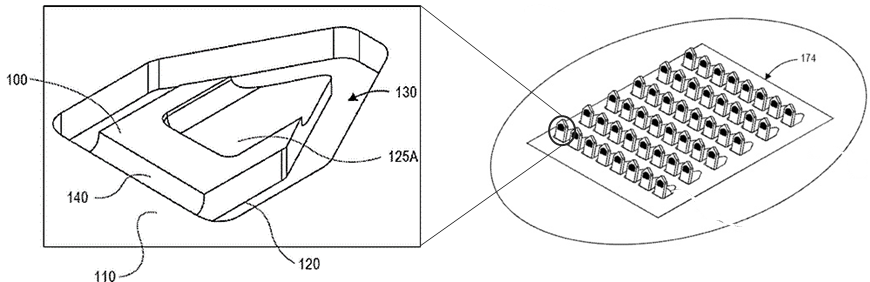
In the meantime, companies such as Verndari Inc., founded in 2015, is developing a vaccine at the UC Davis Medical Center in Sacramento, using the virus “spike” protein to be administer the vaccine via its VaxiPatch™, a dermal patch with a hingeable metal microneedle comprising a reservoir loaded with the vaccine (WO2018026955). This patent family is currently pending in the US, Europe, China and India.
This figure from WO2018026955 illustrates an embodiment of a microtip 100 comprising open reservoir 125A. The microtip 100 comprises a void 130 that is removed during the photochemical or electrochemical etching, die-punching or laser ablation processes used to create the microtip outline 120. A hinged portion 140 is left behind by avoiding complete material removal in this portion (the proximal end) of the microtip, which would detach the microtip 100 from substrate 110.
The VaxiPatch™ technology eliminates the need for refrigeration, allowing for mass manufacturing, which is both cost effective and increases global access to the treatments. It is painless and can potentially be self-administered. Further, preclinical data published in August 2020 have shown that the VaxiPatch™ vaccine system induces stronger immune response, e.g. a 15-fold higher immune response with 1/15 of the dose for the B/Colorado/06/2017 influenza strain.
Considering the numerous advantages of microneedles over standard parenteral injections, it should be expected that at least one of the vaccines currently in development will be approved and used to prevent COVID-19 contamination.
KnowMade’s patent landscape reports about Healthcare.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About our analysts
Matthieu Besse, PhD. Matthieu works for Knowmade as a Patent Attorney and Patent Analyst in the field of Biotechnology and Life Sciences. He holds a PhD in molecular from UCD (University College of Dublin, Ireland), and he is a part-qualified European patent attorney.
About Knowmade
Knowmade is a Technology Intelligence and IP Strategy consulting company specialized in analysis of patents and scientific information. The company helps innovative companies and R&D organizations to understand their competitive landscape, follow technology trends, and find out opportunities and threats in terms of technology and patents.
Knowmade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patents and scientific information into business-oriented report for decision makers working in R&D, Innovation Strategy, Intellectual Property, and Marketing. Our experts provide prior art search, patent landscape analysis, scientific literature analysis, patent valuation, IP due diligence and freedom-to-operate analysis. In parallel the company proposes litigation/licensing support, technology scouting and IP/technology watch service.
Knowmade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Solid-State Sensors/Actuators, Semiconductor Manufacturing, Packaging & Assembly, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
