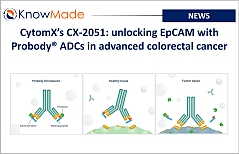
CytomX’s CX-2051: unlocking EpCAM with Probody® ADCs in advanced colorectal cancer
SOPHIA ANTIPOLIS, France – May 30, 2025 │ CytomX Therapeutics announced positive Phase 1 data for its Epithelial Cell Adhesion Molecule (EpCAM) PROBODY® Antibody Drug Conjugate candidate, CX-2051, in advanced Colorectal Cancer (CRC). CytomX Therapeutics: precision oncology through Probody® innovation CytomX Therapeutics is based in South San Francisco, California. It was founded in 2008 by[…]