Our oncology sector brings together world-class patent intelligence and deep scientific insight to support every stage of cancer therapy development. From the identification of novel players to the engineering of innovative therapies (e.g., bispecific antibodies, antibody–drug conjugates, allogeneic CAR), we provide competitive landscaping, freedom-to-operate assessments, and strategic IP guidance tailored to the unique challenges of your oncology programs.
Technical expertise you’ll uncover in our studies
- Patent landscape analysis outlining key players, oncology filing trends, oncology innovation and white-space opportunities
- Real-time patent monitoring to track new filings, oppositions, transfers and litigations in the oncology field
- Scientific literature reviews synthesizing the latest preclinical and clinical data on new trends in oncology
- Freedom-to-operate (FTO) studies and due diligence reports to ensure your candidates can advance without infringement risk
- Patent valuation & portfolio optimization to support licensing, partnering and enforcement strategies
Scroll down to explore our in-depth reports and press releases that will empower your oncology strategy.
Insights
SOPHIA ANTIPOLIS, France – January 30, 2026 │ Roche’s new $570M near‑term licensing deal for MediLink’s YL201 underscores the strategic premium placed on differentiated ADC [...]
SOPHIA ANTIPOLIS, France – November 21, 2025 │ October 21, 2025 – Takeda has entered into a license and collaboration agreement with Innovent Biologics for [...]
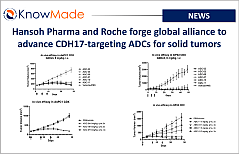
SOPHIA ANTIPOLIS, France – October 24, 2025 │ Hansoh Pharmaceutical has signed a license agreement with Roche to develop HS-20110, a CDH17-targeting antibody-drug conjugate (ADC) [...]
SOPHIA ANTIPOLIS, France – July 10, 2025 │ In July, Regeneron received accelerated approval from the FDA for its bispecific antibody (bsAb) Linvoseltamab (brand name [...]
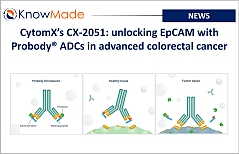
SOPHIA ANTIPOLIS, France – May 30, 2025 │ CytomX Therapeutics announced positive Phase 1 data for its Epithelial Cell Adhesion Molecule (EpCAM) PROBODY® Antibody Drug [...]
SOPHIA ANTIPOLIS, France – April 11th, 2025 │ On March 19th, 2025, a collaboration was announced between Oxford BioTherapeutics (OBT) and Roche to discover novel [...]
Reports
 |
 |
 |
| Bispecific Antibody & Cancer Patent Landscape Analysis |
Allogeneic CAR Patent Landscape Analysis 2023 |
mRNA Cancer Therapies Patent Landscape Analysis 2022 |
Monitor
 |
| Coming soon |