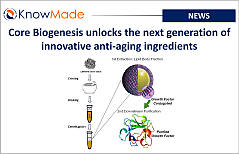
Core Biogenesis unlocks the next generation of innovative anti-aging ingredients
SOPHIA ANTIPOLIS, France – November 04, 2025 │ French biotechnology company Core Biogenesis has recently presented at Cosmetic 360, its oleosome-based growth factor platform promising anti-aging efficacy and supported by a sustainable and scientifically proven approach. Knowmade deciphers the science and the intellectual property behind the innovation. About Core Biogenesis Core Biogenesis is a biotechnology[…]