SOPHIA ANTIPOLIS, France – May 30, 2025 │ CytomX Therapeutics announced positive Phase 1 data for its Epithelial Cell Adhesion Molecule (EpCAM) PROBODY® Antibody Drug Conjugate candidate, CX-2051, in advanced Colorectal Cancer (CRC).
CytomX Therapeutics: precision oncology through Probody® innovation
CytomX Therapeutics is based in South San Francisco, California. It was founded in 2008 by Frederick Gluck, Dr. Nancy Stagliano, and Professor Patrick Daugherty of the University of California Santa Barbara (Department of Chemical Engineering). CytomX is a clinical-stage biopharmaceutical company focused on oncology. The Probody® platform was developed by using technology licensed from the university, which enables for drug designs that can selectively activate in the tumor microenvironment while minimizing drug activity in healthy tissue. Its pipeline comprises various oncology therapeutic candidates such as antibody-drug conjugates (ADCs), T-cell engagers, and immune modulators (e.g., cytokines). The company has developed strategic partnerships with leaders in oncology, including Amgen, Astellas, Bristol Myers Squibb, Regeneron and Moderna.
Probody® therapeutics are designed to fight cancer by exploiting conditions of the tumor microenvironment by localizing treatment in the tumor and limiting activity in healthy tissue. The company designs Probody therapeutics to mask target binding regions. These “novel therapeutics take advantage of the high levels of protease activity in the tumor microenvironment”. When Probody therapeutics encounter active proteases near tumor tissue, the mask is designed to be removed so that the unmasked therapeutic can bind to the tumor target (figure 1). According to the company, Probody therapeutics have the potential to “create or widen therapeutic window (the balance between a therapy’s dose that is effective without causing unacceptable toxicity), enable new combinations of drugs previously not possible due to toxicities and expand the universe of viable therapeutic targets by opening “undruggable” target space”.
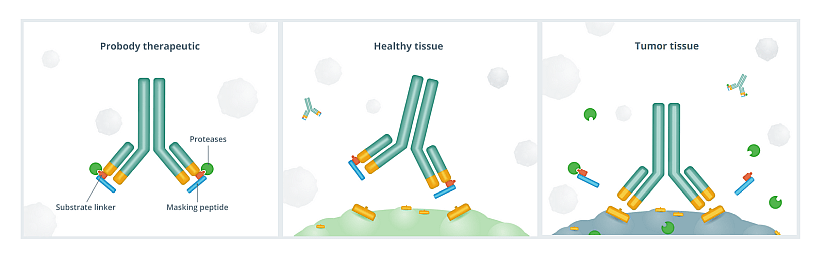
Figure 1: Probody therapeutic. 1- The pioneering antibody prodrug technology. 2- The “masking” peptide is designed to limit the ability of Probody therapeutics to bind to healthy tissue—thereby helping to minimize toxicities. 3- In the tumor environment, protease enzymes are expected to cleave the substrate, removing the “mask” and activating the Probody therapeutic to bind to its target on cancer cells. From CytomX website.
CX-2051: A Next-Generation ADC Targeting EpCAM in Epithelial Cancers
CX-2051 is a conditionally activated ADC directed toward the epithelial cell adhesion molecule (EpCAM), with potential applicability across multiple EpCAM-expressing epithelial cancers. Four decades ago, EpCAM was found to be a tumor antigen in colorectal carcinomas. Due to its frequent and high expression on carcinomas and metastases, EpCAM serves as a prognostic marker, a therapeutic target, and an anchor molecule on circulating and disseminated tumor cells. However, EpCAM plays also a role in adhesion of normal cells which explains the lack of therapy for now.
CX-2051 is an investigational, masked, conditionally activated ADC directed toward EpCAM-expressing epithelial cancers, including CRC, and armed with camptothecin, a topoisomerase-1 inhibitor payload (figure 2). It was developed in collaboration with ImmunoGen, now part of AbbVie.
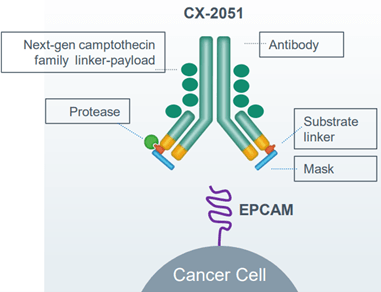
Figure 2: CX-2051 detailed.
First-in-Human study of CX-2051 shows encouraging activity and safety in refractory colorectal cancer
The clinical trial NCT06265688 (CTMX-2051-101) is a Phase 1a open-label study, enrolling patients with advanced solid tumors, with a focus on advanced metastatic colorectal cancer (CRC). The purpose of this first-in-human study is to characterize the safety, tolerability, and antitumor activity of CX-2051 in participants with advanced CRC. The study is comprised of 2 parts. In part 1, there is a dose escalation of CX-2051 to identify the maximum tolerated dose (MTD) of CX-2051. In part 2, safety and tolerability will be further evaluated as well as antitumor activity of CX-2051 in indication-specific expansion cohorts.
The clinical study was initiated in April 2024 with dose escalation proceeding through seven dose levels as of the April 7, 2025 data cutoff. The therapeutic activity of the 2.4 and 4.8 mg/kg doses in single patient dose escalation cohorts was not expected. At the 7.2, 8.6, and 10 mg/kg doses, 23 patients were treated in total, 18 of whom were efficacy evaluable, having had at least one post-baseline tumor assessment as of the data cutoff. CX-2051 was administered on a once every three-week schedule (Q3W). The 25 patients enrolled in the study had previously received a median of 4 prior lines of therapy and all patients had previously been treated with irinotecan. 64% of patients had liver metastases, 64% had KRAS mutations (most commonly occurring hotspot mutations in human cancers and associated with a poor prognosis), and 96% were microsatellite stable. Patients were not preselected based on EpCAM expression levels.
Preliminary data revealed by CytomX:
- Efficacy Results: At the expansion dose levels of 7.2, 8.6 and 10 mg/kg administered every three weeks (Q3W), 18 patients were evaluable for efficacy.
- Overall response rate (ORR): Five of these 18 patients (28 %) achieved confirmed partial Response Evaluation Criteria in Solid Tumors (RECIST) v. 1.1 responses. ORR for currently approved therapies in 3rd line or later CRC are in the low to mid-single digit percentages1. Notably, at the 10 mg/kg dose, 3 of 7 evaluable patients (43%) achieved confirmed partial responses. Across all three dose cohorts, the Disease Control Rate was 94%.
- Durability: At the data cutoff, the median progression free survival was 5.8 months and 10 of 18 patients remained on the treatment.
- Safety Results: CX-2051 was well-tolerated with manageable adverse events, with no observed dose limiting toxicity. Most of treatment related adverse events (TRAEs) were Grade 1 or Grade 2 in severity. The most frequently reported TRAEs were diarrhea, nausea, vomiting, fatigue, anemia, hypokalemia, neutrophil count decrease and neutropenia. Five patients experienced serious TRAEs, but there were no Grade 4 or 5 TRAEs observed. No events of pancreatitis, interstitial lung disease or febrile neutropenia were reported at time of data cutoff.
Dose expansions have been initiated at doses of 7.2, 8.6 and 10 mg/kg Q3W. The selection of the recommended phase 2 dose will be informed by enrolling a total of 20 patients at each dose level.
CytomX builds global IP position around activatable EpCAM ADCs with promising preclinical profiles
CytomX Therapeutics owns 84 patent families (960 documents), filed between 2000 and 2024. The company has a strong worldwide IP strategy, with patent applications in Europe, the USA and Asia. About 73% of its patents are currently alive (pending or granted patents), reflecting that its R&D efforts are still on going to improve the current technologies involving cancer therapies. The strength of its patent portfolio may increase in the coming years because 55% of its live patents are pending applications. CytomX has co-filed several patent families with major players in the pharmaceutical industry such as Immunogen (AbbVie), Amgen or BMS.
There are 2 patent families (26 documents) describing ADC against Epithelial Cell Adhesion Molecule (EpCAM). They were filed in 2019 and 2023 by CytomX in collaboration with Immunogen, acquired by AbbVie in 2024. Both patent applications are published worldwide such as Europe, America (the USA, Canada, Mexico, Brazil), Australia and Asia (Japan, China, Korea, Singapore, India, Taiwan). At the end of March 2025, the Examining Division notified its intention to grant a European patent for the invention EP3873512 (WO2020/086665 – 2019). Before, claims and description were amended several times (December 2021, September 2022, May 2023, September 2023, February 2025). The new claims are now more precise (heavy and light chains with specific sequences). Therefore, they have a more limited scope which is less restrictive for competitors.
The ADC described in the patent family WO2020/086665 is an EpCAM antibody coupled to a maytansinoid compound (antimitotic agent). It is not the ADC CX-2051 which is conjugated to camptothecin. However, this patent family describes also an EpCAM activable antibody, linked to a cytotoxic drug, to treat solid cancers. Studies were performed in CB17 SCID mice bearing NCI- H2110 tumors, a human non-small cell lung cancer sub-cutaneous xenograft model. All treatments were well tolerated at the indicated doses, and no body weight loss was observed. Tumor regressions in the 3 µg/kg regimen started at early time points following ADC administration and resulted in multiple partial regressions as early as 7 days post treatment (figure 3). These data show that treatment with huEpCAM23Gv4.2-DGN549 induces a high incidence of tumor regressions in this tumor model and results in potent anti-tumor activity at doses as low as 1.5 µg/kg.
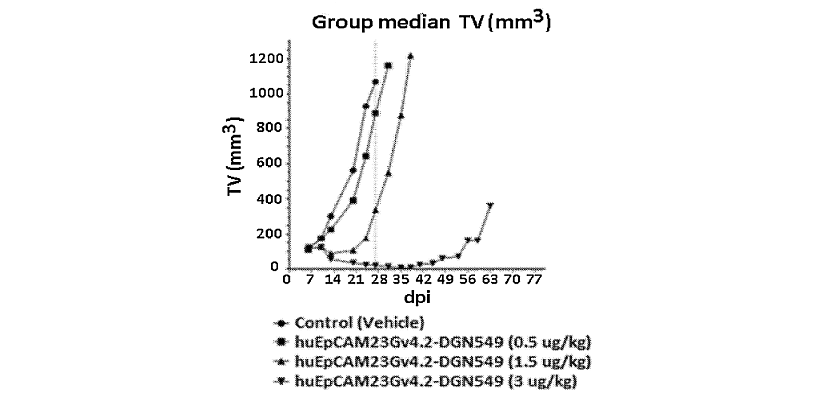
Figure 3: It shows the anti-tumor activity of huEpCAM23Gv4.2-lys-DGN549 in the non-small cell lung cancer xenograft model. The ADC huEpCAM23Gv4.2-DGN549 was highly active at 3 µg/kg, with a tumor growth inhibition value of 1.8%. Furthermore, this group also showed a LCK = 1.77 (log cell kill), qualifying the treatment as active (++), and a 179% increased life span (highly active), demonstrating good tumor growth delay. dpi: days post-inoculation; TV: tumor volume.
The patent family WO2024/015830 relates to an activatable human lgG1 EpCAM antibody. This antibody has eight cysteines covalently bounded to linker-payloads, camptothecin toxin (CPT66). The EpCAM activatable antibodies have relatively low systemic toxicities. In cynomolgus monkeys EpCAM-CPT66 was tolerated up to 60 mg/kg. This was in stark contrast to EpCAM-DM21 , an immunoconjugate comprising the same EpCAM activatable antibody as EpCAM-CPT66 but with the auristatin-based DM21 linker-payload, which was only tolerated up to 6 mg/kg. Tolerability of EpCAM-CPT66 is also favorable when compared to fam-trastuzumab deruxtecan-nxki, where the highest non-severely toxic dose was determined to be 30 mg/kg in cynomolgus monkeys. In another study, unmasked immunoconjugate (EpCAM antibody with conjugated CPT66 linker-payload; EpCAM(-)-CPT66) was also evaluated in a tolerability assay, where it was administered intravenously in cynomolgus monkey at 10 mg/kg at Q2Wx2. The animal made it to scheduled necropsy with mild intervention, mild clinical signs including vomitus and low food consumption. Hematology revealed mild signs of neutropenia and reticulocytosis. At the end of treatment period gross observation was noted in the cecum. Microscopic findings were noted in the lung, Gl tract, and lymphoid tissues. In contrast, there was no test article-related findings at 10 mg/kg dosing of EpCAM-CPT66 (EpCAM activatable antibody) in cynomolgus monkeys.
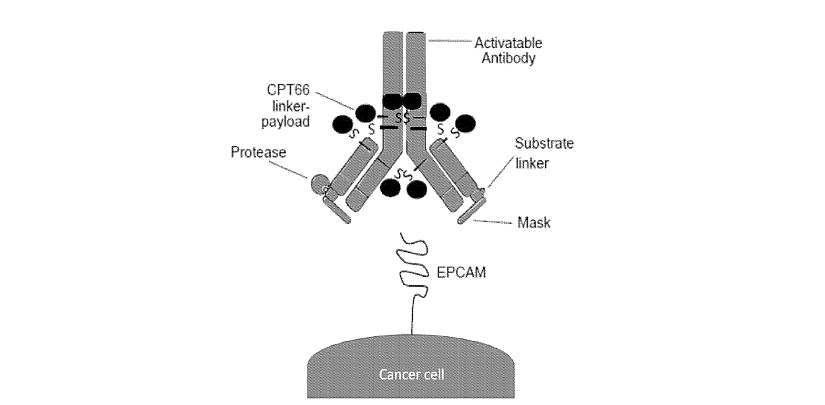
Figure 4: activatable EpCAM antibody immunoconjugate. The ADC comprises an EpCAM antibody, a cleavable substrate linker, and a mask. In an uncleaved (inactive) state, the mask inhibits the binding of the EpCAM antibody to EpCAM. The cleavable substrate linker is cleavable by a protease. Upon cleavage, the mask is released and the antibody is free to bind to EpCAM. There are 8 conjugated camptothecin-derived (CPT66) linker-payloads (shown as ovals in cartoon). The conjugation of the linkerpayloads to the activatable EpCAM antibody is stochastic, with conjugation occurring at the antibody’s inter-chain cysteines.
Strong foundations for the future of CX-2051
CytomX Therapeutics is advancing precision oncology with CX-2051, a novel EpCAM-targeted Probody® ADC showing encouraging early clinical results in advanced colorectal cancer. The favorable safety and efficacy signals underscore its therapeutic promise. With a robust global IP portfolio (84 patent families) and strong industry partnerships, CytomX is well-positioned for future development. Continued clinical progress and patent protection could unlock new treatment options for hard-to-treat epithelial cancers. CX-2051 may represent a new standard in conditionally activated cancer therapies.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About the author
Fabienne Massa, PhD., works for Knowmade in the field of Biotechnology and Life Sciences. She holds a PhD in Molecular and Cellular Biology from the IPMC (Sophia Antipolis, France). She also holds a Master of Business Management from IAE (Nice, France) and she previously worked in the pharmaceutical industry.
About KnowMade
KnowMade is a technology intelligence and IP strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patent information and scientific literature into actionable insights, providing high added value reports for decision makers working in R&D, innovation strategy, intellectual property, and marketing. Our experts provide prior art search, patent landscape analysis, freedom-to-operate analysis, IP due diligence, and monitoring services.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Sensors, Semiconductor Packaging, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
