SOPHIA ANTIPOLIS, France – July 10, 2025 │ In July, Regeneron received accelerated approval from the FDA for its bispecific antibody (bsAb) Linvoseltamab (brand name Lynozyfic). It is intended to treat adult patients with relapsed or refractory (R/R) multiple myeloma (MM) who have received at least 4 prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent and an anti‑CD38 monoclonal antibody. According to Regeneron, “Linvoseltamab is the 1st FDA-approved BCMAxCD3 bsAb that can be dosed every 2 weeks starting at week 14, and every 4 weeks if a very good partial response or better is achieved following completion of at least 24 weeks of therapy”. The company and its patent portfolio have been previously described in a KnowMade article “Regeneron Strengthens Odronextamab Patent Portfolio While Adjusting Approval Strategy”.
Linvoseltamab (REGN5458), a fully human BCMA×CD3 bsAb for R/R multiple myeloma
Linvoseltamab is a fully human BCMAxCD3 bsAb designed to bridge B-cell maturation antigen (BCMA) on MM cells with CD3-expressing T cells to facilitate T-cell activation and cancer-cell killing. It was invented using Regeneron’s VelocImmune® technology which utilizes a proprietary genetically engineered mouse platform endowed with a genetically humanized immune system to produce optimized fully human antibodies. Lynozyfic is cautioned against cytokine release syndrome and neurotoxicity.
The FDA initially rejected the drug in August 2024 due to manufacturing issues at a third-party fill/finish site. However, on July 2, the FDA granted accelerated approval to Regeneron’s bispecific antibody Linvoseltamab for the treatment of patients with R/R MM. Lynozyfic is also approved in the European Union to treat adults with R/R MM after at least 3 prior therapies, including a proteasome inhibitor, an immunomodulatory agent and an anti-CD38 monoclonal antibody, and have demonstrated disease progression on the last therapy.
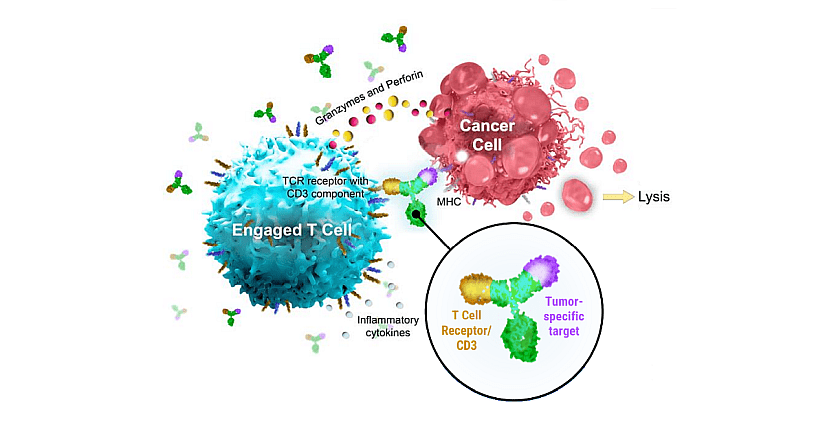
Figure 1: Schematic representation of bsAb from Regeneron. For Linvoseltamab, the tumor-specific target (purple) is BCMA binding sites. MHC, major histocompatibility complex; TCR, T-cell receptor. From “Regeneron Corporate presentation – February 2024”.
Evolution of Linvoseltamab clinical development across 12 myeloma studies
There are 12 studies, identified on ClinicalTrials.gov, describing Linvoseltamab for myeloma treatment (table below). For all of them, Regeneron is the sponsor or the collaborator.
Between January 2019 and mid-2025, twelve Phase 1/2, Phase 2 and Phase 3 trials of Linvoseltamab have launched, reflecting a clear shift from first-in-human dose-escalation studies toward combination regimens and maintenance strategies. Early trials established safety and optimal dosing in relapsed/refractory multiple myeloma, paving the way for studies pairing Linvoseltamab with daratumumab, carfilzomib or lenalidomide and even another bsAb from Regeneron to enhance efficacy. Simultaneously, “window-of-opportunity” and IMMUNOPLANT trials are exploring activity in newly diagnosed, treatment-naïve patients, while a dedicated maintenance study aims to prolong minimal residual disease negative status. Nearly all studies are actively recruiting, underscoring robust interest in this BCMA×CD3 bispecific across diverse lines of therapy and clinical settings.
| NCT Number | Study Title | Study Status | Conditions | Interventions | Phases | Start Date |
| NCT03761108 | Phase 1/2 Study of REGN5458 in Adult Patients With Relapsed or Refractory Multiple Myeloma | Recruiting | Multiple Myeloma | Linvoseltamab | Phase 1 Phase 2 | 23/01/2019 |
| NCT05164250 | Compassionate Use (CU) of REGN5458 for Patients With Relapsed or Refractory Multiple Myeloma (MM) | Available | Multiple Myeloma | Linvoseltamab | / | 20/12/2021 |
| NCT05137054 | A Study to Examine the Effects of Novel Therapy Linvoseltamab in Combination With Other Cancer Treatments for Adult Patients With Multiple Myeloma That is Resistant to Current Standard of Care Treatments | Recruiting | Multiple Myeloma | Linvoseltamab | Daratumumab | Carfilzomib | Lenalidomide, … | Phase 1 | 17/08/2022 |
| NCT05730036 | A Trial to Learn How Well Linvoseltamab Works Compared to the Combination of Elotuzumab, Pomalidomide and Dexamethasone for Adult Participants With Relapsed/Refractory Multiple Myeloma | Recruiting | Multiple Myeloma (Relapsed Refractory) | Linvoseltamab | Elotuzumab | Pomalidomide | Dexamethasone | Phase 3 | 18/09/2023 |
| NCT05828511 | A Window of Opportunity Trial to Learn if Linvoseltamab is Safe and Well Tolerated, and How Well it Works in Adult Participants With Recently Diagnosed Multiple Myeloma Who Have Not Already Received Treatment | Recruiting | Multiple Myeloma | Linvoseltamab | Phase 1 Phase 2 | 19/12/2023 |
| NCT05955508 | A Proof-of-Concept Trial to Study the Safety and Activity of Linvoseltamab in Participants With Smoldering Multiple Myeloma at High Risk of Developing Multiple Myeloma | Recruiting | SmolderingMultiple Myeloma | Linvoseltamab | Phase 2 | 30/01/2024 |
| NCT06376526 | IMMUNOPLANT for Newly Diagnosed Multiple Myeloma | Recruiting | Multiple Myeloma | Linvoseltamab | Phase 2 | 21/08/2024 |
| NCT06140524 | A Proof-of-Concept Study to Learn Whether Linvoseltamab Can Eliminate Abnormal Plasma Cells That May Lead to Multiple Myeloma in Adult Patients With High-Risk Monoclonal Gammopathy of Undetermined Significance or Non-High-Risk Smoldering Multiple Myeloma | Recruiting | Monoclonal Gammopathy of Undetermined Significance | Smoldering MM | Linvoseltamab | Phase 2 | 16/09/2024 |
| NCT06669247 | A Study to Assess the Safety and Anti-Tumor Activity of REGN7945 in Combination With Linvoseltamab in Adult Participants With Relapsed/Refractory Multiple Myeloma | Recruiting | Multiple Myeloma (Relapsed Refractory) | Linvoseltamab | REGN7945+Linvoseltamab | Phase 1 Phase 2 | 11/12/2024 |
| NCT06932562 | A Study to Learn How Safe and How Well Linvoseltamab Works Compared to Standard Treatment in Adult Patients With Multiple Myeloma Who Are Not Eligible for Transplant | Not Yet Recruiting | Multiple Myeloma | Linvoseltamab | Daratumumab | Lenalidomide | Dexamethasone | Phase 3 | 17/04/2025 |
| NCT06910124 | Linvoseltamab in Addition to Lenalidomide (L2) During Maintenance Therapy of NDMM to Deepen Responses or Redrive MRD Negativity After Relapse | Not Yet Recruiting | Multiple Myeloma | Linvoseltamab | Lenalidomide | Phase 2 | 01/08/2025 |
| NCT07009899 | BCMA Bispecific Antibody Therapy for Post-BCMA CAR T-Cell Therapy Relapse (RECLAIM) | Not Yet Recruiting | Multiple Myeloma | Linvoseltamab | Phase 2 | 01/09/2025 |
Table 1: Clinical trials for Myeloma treatment with Linvoseltamab.
Efficacy and adverse-event profile of Linvoseltamab in the LINKER-MM1 R/R MM study
The ongoing, open-label, multicenter Phase 1/2 dose-escalation and dose-expansion LINKER-MM1 trial (NCT03761108) is investigating Linvoseltamab in more than 300 enrolled patients with R/R MM. The Phase 1 intravenous dose-escalation portion of the trial assessed safety, tolerability and dose-limiting toxicities (nine dose levels) and explored different administration regimens. A subcutaneous Phase 1 portion and an intravenous dose expansion portion Phase 2 are ongoing.
Linvoseltamab was approved by the FDA based on the results of the pivotal Phase 1/2 LINKER-MM1 trial that investigated it for R/R MM. For 80 patients of the study:
- The objective response rate is 70% and 45% achieve a complete response or better.
- The median time to first response is 0.95 months (range: 0.5 to 6 months).
- The median duration of response (DoR) was not reached. Among responders who had a median follow-up of 13 months, the estimated DoR was 89% at 9 months and 72% at 12 months.
- The most common adverse reactions (≥20%) in the safety population of LINKER-MM1 (n=117) were musculoskeletal pain, CRS, cough, upper respiratory tract infection, diarrhea, fatigue, pneumonia, nausea, headache and dyspnea.
- The most common Grade 3 or 4 laboratory abnormalities (≥30%) were decreased lymphocyte count, decreased neutrophil count, decreased hemoglobin and decreased white blood cell count.
Two complementary patents: BCMA×CD3 bsAb and its dosage regimen
Regeneron has 49 patent families related to bispecific antibodies for oncology treatment, published between 2010 and 2024 (see KnowMade’s report: Bispecific Antibody & Cancer Patent Landscape Analysis).
The patent family WO2020/018820, published in 2020, includes granted patents in Europe, the USA, Japan, China, and other countries, as well as pending applications in Canada, Australia, Brazil, Korea, and additional jurisdictions. It provides bsAbs that bind to both BCMA and CD3, to treat myeloma. In vitro assays, such as binding assays and cytotoxic activity measurements, confirm that these bispecific antibodies activate T cells and mediate the lysis of BCMA-positive cell lines. In vivo efficacy is demonstrated in mouse xenograft models where single or repeat dosing at 0.04 to 4 mg/kg achieves significant tumor growth inhibition. Anti-BCMA x anti-CD3 bsAbs reduce the size and prevent growth of established BCMA-expressing tumors, in a dose-dependent manner (figure 2). These bispecific antibodies also reduce tumor burdens to background levels in vivo.
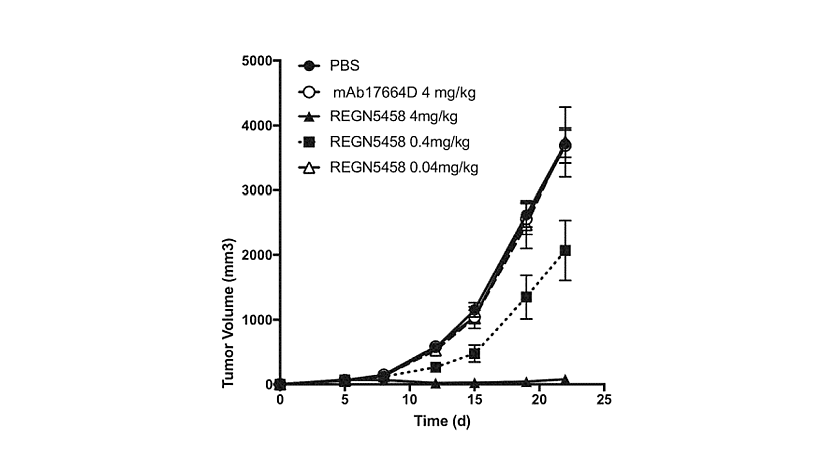
Figure 2: therapeutic dose-dependent tumor inhibition in vivo by anti-BCMA x anti-CD3 bsAbs (REGN5458).
Immunodefcient NOD.Cg-PrkdcscidII2rgtm1Wjl/SzJ (NSG) mice were subcutaneously implanted with a mixture of 10×106 BCMA-expressing NCI-H929 human multiple myeloma tumor cells and 0.5×106 human peripheral blood mononuclear cells isolated from a normal, healthy donor. On day 5, the mice (n=7 8 per group) were then administered a PBS vehicle control, a CD3-binding control bispecifcAb (mAb17664D) at a dose of 4 mg/kg, a BCMAxCD3 (REGN5458) bsAb at doses of either 4 mg/kg, 0.4 mg/kg, or 0.04 mg/kg. The mice were administered these Abs twice per week for a total of seven doses, and tumor growth was assessed over 55 days. While BCMA+ NCI-H929 tumors grew similarly in the vehicle and CD3-binding control-treated mice, BCMAxCD3 Abs that were tested shrank established tumors and prevented the growth of tumors in a dose-dependent manner in vivo.
One year later, the patent family WO2021/113701 was published. It now includes granted patents in the USA, Japan, and China, as well as pending applications in Europe, Canada, Australia, South Korea, and other jurisdictions. It describes methods for treating multiple myeloma (R/R) using bsAbs that bind to both BCMA and CD3. The subject received at least three prior therapies for multiple myeloma. The bispecific antibody is administered to the subject in a dosing regimen comprising the administration of a single dose of 5 mg of the bsAb during week one, a single dose of 25 mg during week two and a single dose of 200 mg during week three of the dosing regimen.
In summary, Linvoseltamab represents a landmark advancement in the treatment of heavily pretreated multiple myeloma, combining a bispecific antibody targeting BCMA and CD3 complemented by a convenient administration schedule. Its accelerated FDA approval—supported by robust safety and efficacy data from the pivotal LINKER-MM1 trial—validates the clinical potential of T-cell–redirecting therapies in relapsed/refractory multiple myeloma. Across many ongoing clinical studies, Linvoseltamab continues to demonstrate deep, durable responses with a manageable toxicity profile. Noteworthy, the two complementary patent families afford very specific protection for both the BCMA×CD3 bsAb (structure, optimized VH/VL sequences, preclinical efficacy) and its optimized dosing regimen for the treatment of multiple myeloma (dosages, administration regimens, drug combinations). Together, they provide comprehensive protection from molecular design to clinical applications, and the pending applications in key regions are expected to be granted soon, ensuring a broad, global IP shield. Moreover, according to Sundar Jagannath, M.D., Network Director of the Center of Excellence for Multiple Myeloma at Mount Sinai (New York) “Lynozyfic has a convenient response-adapted dosing regimen, which provides the potential to extend time between doses. This is a significant patient-centric advancement that could help reduce treatment burden.” Together, these developments underscore a new era of targeted immunotherapy for BCMA-expressing malignancies and pave the way for broader exploration of bispecific antibodies in oncology.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About the author
Fabienne Massa, PhD., works for KnowMade in the field of Biotechnology and Life Sciences. She holds a PhD in Molecular and Cellular Biology from the IPMC (Sophia Antipolis, France). She also holds a Master of Business Management from IAE (Nice, France) and she previously worked in the pharmaceutical industry.
About KnowMade
KnowMade is a technology intelligence and IP strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patent information and scientific literature into actionable insights, providing high added value reports for decision makers working in R&D, innovation strategy, intellectual property, and marketing. Our experts provide prior art search, patent landscape analysis, freedom-to-operate analysis, IP due diligence, and monitoring services.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Sensors, Semiconductor Packaging, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
