SOPHIA ANTIPOLIS, France – November 21, 2025 │ October 21, 2025 – Takeda has entered into a license and collaboration agreement with Innovent Biologics for the development, manufacturing and commercialization of two late-stage oncology medicines, worldwide outside of Greater China. First, there is IBI363, a PD-1/IL-2α-bias bispecific antibody fusion protein, evaluated in non-small cell lung and colorectal cancers. Then, there is IBI343, a Claudin 18.2 antibody-drug conjugate (ADC), evaluated in gastric and pancreatic cancers. Takeda will also receive an exclusive option to license global rights outside of Greater China for IBI3001, a potential first-in-class bispecific ADC designed to target both EGFR and B7H3.
From Suzhou to Tokyo: Innovent and Takeda Join Forces in Next-Generation Cancer Therapies
Innovent, a Chinese biopharmaceutical company founded in 2011, discovers, develops, manufactures and commercializes medicines for therapies in cancer, cardiovascular and metabolic, autoimmune and eye diseases. The company owns 16 patent families describing ADC against cancer and has set up more than 120 clinical trials in oncology.
Takeda is a R&D biopharmaceutical company headquartered in Japan, founded in 1781. It develops treatments in its core therapeutic and business areas, including gastrointestinal and inflammation, rare diseases, plasma-derived therapies, oncology, neuroscience and vaccines. The pipeline of Takeda oncology (originally Millennium Pharmaceuticals, a fully owned subsidiary of Takeda since 2008) focuses on thoracic, gastrointestinal and hematologic cancers and their core modalities include ADCs, complex biologics and small molecules. The company has 15 patent families in ADC & Cancer area and has set up more than 340 clinical trials in oncology.
IBI343: A Next-Generation Claudin 18.2-Targeted ADC for Gastrointestinal Cancers
IBI343 is a monoclonal antibody-drug conjugate that targets Claudin 18.2-expressing tumor cells. Claudin 18.2 is typically found in healthy stomach, but it is abnormally expressed on the surface of gastric and pancreatic cancer cells, making it a target for new cancer therapies. These cancers have some of the lowest 5-years survival rates.
The ADC is an anti-Claudin 18.2 antibody linked to a cytotoxic agent Exatecan, a topoisomerase I inhibitor (TOPO1i). In June 2024, the US FDA has granted Fast Track Designation to IBI343 for the treatment of advanced unresectable or metastatic pancreatic ductal adenocarcinoma (PDAC) that has relapsed and/or is refractory to one prior line of therapy.
Ongoing Clinical Evaluation of IBI343 in Gastric and Pancreatic Cancers
IBI343 is currently being evaluated in a comprehensive clinical program covering gastric, gastroesophageal junction (GEJ), and pancreatic cancers.
The first-in-human Phase 1a/1b study (NCT05458219) investigates IBI343 as monotherapy in patients with advanced solid tumors, including CLDN18.2-positive gastric and GEJ adenocarcinomas. The trial includes a dose-escalation phase followed by expansion cohorts. Preliminary results from this study, recently published in Nature Medicine (July 2025), demonstrated a manageable safety profile and promising efficacy in G/GEJ adenocarcinoma. A total of 127 patients were enrolled. Despite the higher objective response rate (ORR) and longer progression-free survival (PFS) observed at a dose of 8 mg kg−1, the safety profile at a dose of 6 mg kg−1 was more favorable with significantly lower rates of treatment interruption and discontinuation. The presence of dose-limiting toxicities was reported in two of six participants, including one with myelosuppression (grade 4) and one with both neutropenia (grade 4) and febrile neutropenia (grade 3). The study resulted in a minimal number of gastrointestinal adverse events (grade ≥ 3) and no reports of interstitial lung disease.
Building on these results, Innovent initiated two Phase 3 trials to further evaluate IBI343 monotherapy. The G-HOPE-001 study (NCT06238843) is a multicenter randomized trial comparing IBI343 to investigator’s choice of therapy in previously treated, HER2-negative, CLDN18.2-positive gastric or GEJ adenocarcinoma. A parallel Phase 3 study (NCT07066098) explores IBI343 in patients with locally advanced or metastatic pancreatic cancer after at least two prior lines of therapy. The primary objective of this study is to determine Overall Survival (OS) of IBI343 plus best supportive care (BSC) compared with placebo plus BSC. Both studies are currently recruiting, and no interim results have been released as of November 2025.
Combination regimens are also under active investigation. The DRAGON-15 study (NCT07025889) is a multicenter Phase 1b/2 trial assessing IBI343 combined with Sintilimab (PD-1 inhibitor) and chemotherapy as first-line treatment for CLDN18.2-positive, HER2-negative gastric/GEJ adenocarcinoma. A phase 2 study has the same development framework but without chemotherapy (NCT06321913). Then, another Phase 2 study (NCT06770439) evaluates IBI343 in combination with chemotherapy, AG regimen (albumin paclitaxel with gemcitabine), in advanced pancreatic cancer. No preliminary data is available.
Together, these six clinical trials illustrate a coherent strategy to position IBI343 as a fundamental therapeutic option for gastrointestinal and pancreatic tumors expressing CLDN18.2, either as monotherapy or in combination to enhance clinical benefit.
Intellectual Property Supporting IBI343 Development
Innovent owns 16 patent families (55 documents) in the area, filed between 2022 and 2025. The company has a strong worldwide IP strategy, with patent applications in Europe, the USA and Asia. 100% of its patents are currently alive (pending or granted patents), reflecting that its R&D efforts are still on going to improve the current technologies involving cancer therapies.
To protect IBI343 invention, four recent international patent applications filed by Innovent Biologics outline a robust strategy to protect the Claudin 18.2-targeted ADC technology, covering its composition, therapeutic uses, formulation and combination therapy.
First, the ADC in the patent family WO2023/109953, published in 2023, described an antibody that binds to Claudin 18.2, a linker and a payload such as Topoisomerase 1 inhibitor (e.g., exatecan, deruxtecan) or tubulin binders (e.g., MMAE, DM1). In vivo anti-tumor effect of CLDN18.2 antibodies were tested. First, in NOD-SCID mice with human pancreatic cancer (cancer cells DAN-G-hCLDN18.2), were administered at a dose of 10 mg/kg on days 5, 9, 12, and 16 after tumor cell inoculation. Second, ADCs were administered in NOG mice with human gastric cancer (PBMC cells – Allcells) at a dose of 10 mg/kg on days 1, 5, 8, and 12 after tumor cell inoculation. The tumor volume and body weight of the mice was monitored 2-3 times per week. Both HB37A6 (ADC treatment) and Zmab (control antibody Zolbetuximab) inhibited tumor growth in human pancreatic cancer, with tumor growth inhibition (TGI) values of 28% and 24%, respectively (figure 1a). HB37A6 showed better anti-tumor effect than the control antibody Zmab in human gastric cancer NUGC-4 mouse models, with TGI values of 31% and 0%, respectively (figure 1b).
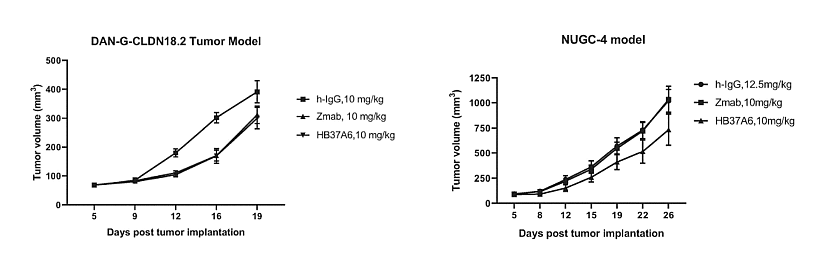
Figure 1: Anti-tumor effect of the HB37A6 antibody in the a) pancreatic cancer mouse model and b) gastric cancer mouse model.
Secondly, the patent family WO2024/255880, published in December 2024, details a method of treating solid tumors by administering a Claudin 18.2-targeting ADC. In examples, the Phase 1a/1b clinical study (NCT05458219) as well as the Phase 3 clinical trial (NCT06238843) are described. During dose escalation, 6 dose levels of the ADC (0.3/1/3/6/8/10 mg/kg intravenous Q3W) were evaluated. Selected dose levels were expanded in gastric/gastro-esophageal junction adenocarcinoma pts with positive CLDN18.2 expression, defined as ≥1% tumor cells with membranous staining of any intensity (1+/2+/3+) by immunohistochemistry.
Third, a pharmaceutical formulation is detailed in the patent family WO2024/255879, published in December 2024. The formulation comprised about 20mg/ml of the ADC, about 20 mM of buffer (histidine +/- histidine hydrochloride), about 8% (w/v) of stabilizer (sucrose, trehalose, or a combination of sucrose and mannitol) and about 0.02% (w/v) of polysorbate 80. The pH of the formulation is about 6.5.
Finally, WO2025/077815 provides a method of using a Claudin18.2-targeted ADC in combination with antibody drugs and/or chemotherapy drugs for treating cancer. A clinical trial is detailed in the description. It is a phase II study to assess the safety, tolerability, pharmacokinetics and efficacy of the ADC in combination with treatment of advanced malignant solid tumor subjects, including Part 1 (Security import phase) and Part 2 (POC pilot phase). In part 2, 4 cohorts were established: CLDN18.2-positive advanced GEJ adenocarcinoma with first-line standard-care failure or intolerance G/GEJ AC subjects; HER2-negative, CLDN18.2-positive advanced G/GEJ without prior systemic therapy AC subjects; Past treatment failure or intolerance with gemcitabine-based regimens, CLDN18.2-positive PDAC subjects; and 80 patients with advanced CLDN18.2-positive PDAC who had not undergone systemic therapy after the safe introduction phase.
IBI3001: A First-in-Class Bispecific ADC Targeting EGFR and B7-H3 for Solid Tumors
IBI3001 is a potential first-in-class bispecific antibody-drug conjugate that comprises a bispecific antibody targeting EGFR and B7H3 antigens and a topoisomerase 1 inhibitor payload, the exatecan, an analog of camptothecin. Both EGFR and B7H3 are co-expressed in multiple solid tumors. Previously, additionally to the cytotoxic effects of the payload and strong bystander killing effect, IBI3001 has been found to inhibit EGFR signaling.
As part of the agreement, Innovent will be solely responsible for the clinical development of IBI3001 before exercising the option to license. Takeda will develop, manufacture, and commercialize IBI3001 worldwide, outside of Greater China if it decides to take the option.
IBI3001 Enters Clinical Development: Phase 1 Study and Preclinical Insights
This is a Phase 1 (NCT06349408) multicenter, multi-regional, open-label, first-in-human study of IBI3001 in participants with unresectable, locally advanced or metastatic solid tumors, initiated in January 2025. The purpose of this study is to identify the maximum tolerated dose (MTD) / recommended phase II dose (RP2D) of IBI3001, and to explore the preliminary efficacy of IBI3001, which is proposed to be administered by intravenous infusion. No human preliminary data is available.
However, animal data have been mentioned during the conference of the American Association for Cancer Research Annual Meeting 2024 (Jian Guan et al., “IBI3001: A potentially first-in-class site-specifically conjugated B7-H3/EGFR bispecific ADC for multiple solid tumors”; Proceedings of the American Association for Cancer Research Annual Meeting 2024). According to the author, IBI3001 has a favorable PK profile in BALB/c mice with a half-life of 282 hours, and it is well-tolerated in cynomolgus monkeys up to 90 mg/kg/week.
Intellectual Property Supporting IBI3001 Development
In support of IBI3001’s development, 2 recent international patent applications are filed by Innovent Biologics in December 2024.
Patent family WO2025/131054 discloses ADC antibodies targeting EGFR and B7-H3, their conjugation to cytotoxic payloads, relevant compositions, and methods of use in oncology and diagnostics. In vivo effect of EGFR – B7-H3 antibodies in mouse tumor models were tested. First, NCI-H1975 (non-small cell lung cancer human cells – NSCLC) were inoculated into the right abdomen of CB17/SCID mice. On day 7, mice were grouped and administrated via tail vein, with a dosing cycle: once a week, two administrations in total. Both 1 and 3mg/kg of BS01-NT3 (recombinant humanized anti EGFR/B7-H3 bispecific antibody conjugated to the topoisomerase I inhibitor) showed in vivo anti-tumor activity (figures 2a and b). On the 36th day, the TGI of mice was calculated. At a dose of 3mg/kg, the average tumor size was 66 mm3 and the TGI was >100%. The analysis of weight changes in mice for the 1 and 3mg/kg groups showed that BS01-NT3 had good safety. Second, JIMT-1 cells (human breast cancer cell line) were inoculated into the right abdomen of CB17/SCID mice. On day 8, mice were grouped and administrated via tail vein, with a dosing cycle: once a week, two administrations in total. BS01-NT3 3mg/kg showed good in vivo anti-tumor activity, with an average tumor size of 62 mm3 and a TGI of >100% (figures 2c and d). The analysis of weight changes in mice for the 1mg/kg and 3mg/kg groups showed that BS01-NT3 had good safety.
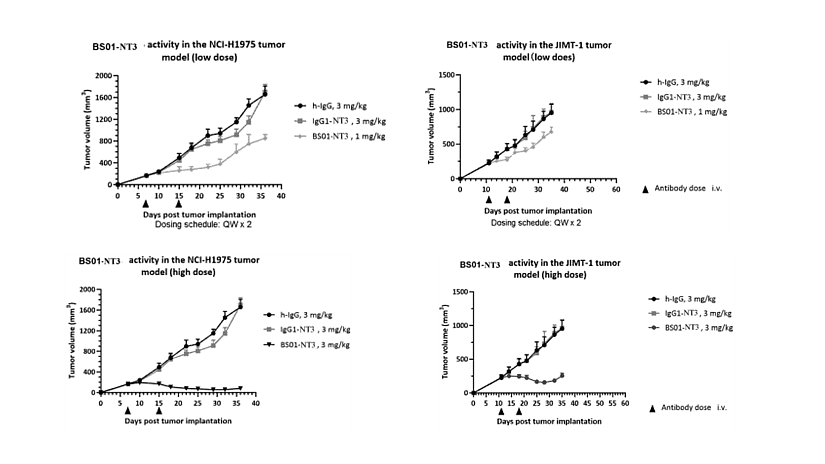
Figure 2: In vivo efficacy in mouse tumor model. a) and b) show in vivo anti-tumor activity of BS01-NT3 in NCI-H1975 (NSCLC) CDX mouse tumor model. c) and d) show in vivo anti-tumor activity of BS01-NT3 in JIMT-1 (BCAR) CDX mouse tumor model. IgG1-NT3 (also referred to as Isotype control NT3).
Then, the patent family WO2025/131053 describes a bispecific ADC which simultaneously bind EGFR and B7-H3, pharmaceutical compositions comprising such ADCs, and their therapeutic and diagnostic uses in cancers. In the description, three bispecific antibodies were obtained by screening, which were numbered as bispecific antibody molecules: Hz5C2.9/Zalu bsAb, Hz19A2.25/Zalu bsAb, and Hz20G5.26/Zalu bsAb. In vitro activity demonstrated that ADC Hz20G5.26/Zalu bsAb-Exatecan had proliferation inhibitory activity by blocking EGFR signals and ADC inducing target tumor cell death activity and ADC bystander effect. In vivo, the effect of this ADC was tested in mouse tumor models, with various cancer cell lines:
- NCI-H1975 (NSCLC): on day 36, for a dose of 1mg/kg, the TGI rate was 104%; for a dose of 3mg/kg, and the TGI rate was 109% (figure 3a).
- BxPC3 (pancreatic adenocarcinoma): on day 53, for a dose of 1mg/kg, the TGI rate was 79%; for a dose of 3mg/kg, the TGI rate was 83%; for a dose of 10mg/kg, TGI rate was 103% (figure 3b).
- NCI-H508 (colorectal adenocarcinoma): on day 53, for a dose of 1mg/kg, the tumor growth inhibition (TGI) rate was 121%, and complete tumor elimination occurred in 3 of 6 mice (figure 3c).
- JIMT-1 (human breast cancer cell line): on day 28, for 1mg/kg dose, the TGI rate was 59%; for 3mg/kg dose, the TGI rate was 107%; for 10mg/kg dose, the TGI rate was 108%, and complete tumor elimination occurred in one of five mice (figure 3d).

Figure 3: In vivo efficacy of Hz20G5.26/Zalu bsAb-Exatecan in mouse model. a) H1975, b) BxPC3, c) H508 and d) JIMT-1 CDX mouse tumor model.
Patent-Driven Innovation at the Core of Takeda and Innovent’s Oncology Alliance
The collaboration between Takeda and Innovent Biologics marks a significant milestone in the evolution of next-generation oncology therapies. By combining Innovent’s innovative antibody–drug conjugate (ADC) platforms and Takeda’s global development and commercialization expertise, the partnership has the potential to accelerate the availability of breakthrough medicines for patients with hard-to-treat solid tumors. Both companies have built a strong foundation of intellectual property, reflecting a shared commitment to scientific excellence and long-term innovation. In parallel, each company is expected to continue expanding its own ADC portfolio, Innovent by leveraging its discovery platforms and China-based clinical engine, and Takeda by integrating licensed ADC assets into its global oncology network – thereby multiplying opportunities for new combinations, new targets and new indications. With patent families protecting key assets such as IBI343 (anti-Claudin 18.2 ADC) and IBI3001 (anti-EGFR/B7-H3 ADC), and an expanding pipeline of clinical programs across major cancer indications, Takeda and Innovent are redefining the landscape of targeted therapies. This collaboration underscores the growing global momentum behind ADC technologies and represents a forward-looking model for advancing precision oncology through strategic partnerships and robust innovation.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About the author
Fabienne Massa, PhD., works for KnowMade in the field of Biotechnology and Life Sciences. She holds a PhD in Molecular and Cellular Biology from the IPMC (Sophia Antipolis, France). She also holds a Master of Business Management from IAE (Nice, France) and she previously worked in the pharmaceutical industry.
About KnowMade
KnowMade is a technology intelligence and IP strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patent information and scientific literature into actionable insights, providing high added value reports for decision makers working in R&D, innovation strategy, intellectual property, and marketing. Our experts provide prior art search, patent landscape analysis, freedom-to-operate analysis, IP due diligence, and monitoring services.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Sensors, Semiconductor Packaging, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
