SOPHIA ANTIPOLIS, France – February 4th, 2025 (last update 10th April, 2025) │ Advancements in oncology have consistently pushed the boundaries of therapeutic innovation, particularly in the realm of immunotherapy. BioNTech’s BNT327, a bispecific antibody targeting vascular endothelial growth factor (VEGF) and Programmed death-ligand 1 (PD-L1), represents a cutting-edge approach to treating difficult cancers such as triple-negative breast cancer (TNBC). This article explores the companies behind the development of BNT327, the intellectual property protecting its innovation, and the promising clinical results that position it as a potential game-changer in cancer therapy.
BioNTech & Biotheus
BioNTech SE, founded in 2008, is a leading biotechnology company headquartered in Mainz, Germany. Renowned for its innovative approach to immunotherapy, the company specializes infectious disease vaccines and in individualized cancer therapies. Its broad portfolio of oncology product candidates includes individualized and off-the-shelf mRNA-based therapies, innovative chimeric antigen receptor T cells, several protein-based therapeutics, including bispecific immune checkpoint modulators, targeted cancer antibodies and antibody-drug conjugate therapeutics. One of its key areas of focus is the development of bispecific antibodies (bsAbs) that simultaneously target two distinct antigens, providing a more comprehensive therapeutic effect. BioNTech’s acquisition of Biotheus has strengthened its pipeline in oncology.
Biotheus Inc., founded in 2018 in China, focuses on bispecific antibodies in oncology and for patients with inflammatory disease. It has pioneered several bsAbs and multi-specific antibodies, including the anti-VEGF / anti-PD-L1 molecule that underpins BNT327.
In November 2024, BioNTech announced its acquisition of Biotheus to boost its oncology strategy. For BioNTech, it provides full global rights to BNT327, with potential to replace current checkpoint inhibitor standard of care treatments for solid tumors. It aims to further strengthen its capabilities to develop, manufacture and commercialize next-generation bsAbs and novel treatment combinations. Moreover, the two companies plan to initiate multiple registrational trials with BNT327 in late 2024 and 2025.
Details of the BNT327 Clinical Trial
About ten clinical studies are underway on BNT327 (BioNTech) / PM8002 (Biotheus). Only trials with preliminary results are detailed.
Breast cancer preliminary results
The study was first posted in August 2024 and is still recruiting (NCT06449222). This study is a Phase II, multi-site, randomized, open-label clinical study to evaluate the safety, efficacy, and pharmacokinetics of BNT327 at two dose levels in combination with chemotherapeutic agents in the first- and second-line treatment of participants with locally advanced/metastatic triple-negative breast cancer (mTNBC). The study plans to randomize or assign eligible participants into two cohorts, i.e., Cohort 1 and Cohort 2. In Cohort 1, participants will be randomized to two treatment arms investigating two dose levels of BNT327 in combination with Nab-paclitaxel. Cohort 2 will not begin until the appropriate dose to move forward has been determined from Cohort 1. After this, the arms in Cohort 2 exploring different chemotherapy combinations will begin to enroll. Participants in Cohort 2, Arm 1 will receive the optimal dose of BNT327 in combination with paclitaxel. Participants in Cohort 2, Arms 2 and 3, will receive the equivalent dose of BNT327 administered once every 3 weeks in combination with gemcitabine plus carboplatin (Arm 2), or eribulin (Arm 3).
The preliminary data of BNT327 bsAbs presented at a recent conference (2024 San Antonio Breast Cancer Symposium) reveal promising outcomes:
- Overall response of 73.8% at a median follow-up of 18.1 months.
- Disease Control Rate: 95.2% of participants exhibited stable disease or better.
- Matured median Progression-Free Survival: 13.5 months.
- Safety: All patients experienced treatment-related adverse events, nearly 60% of which were grade 3 and 4 in severity, including neutropenia, leukocytopenia, anemia and proteinuria. None of the patients died due to side effects.
Lung cancer data, presented at the 2025 European Lung Cancer Congress
Several clinical trials are underway on lung cancer, including 5 set up by Biotheus and 5 by BioNTech. Three studies of Biotheus are active, not recruiting, and preliminary results have been revealed for two of them.
NCT05844150 study, phase 2, evaluates the efficacy and safety of BNT327 in combination with etoposide and platinum, in first line (1L) treatment of extensive-stage small cell lung cancer (SCLC). The first part is single-arm study, 50 participants were enrolled, and recruitment was completed. The second part is a randomized, double-blind study, active controlled design, which will be integrated to another global III study (NCT06712355). Preliminary results showed that BNT327 combined with platinum-based chemotherapy demonstrated encouraging efficacy. Moreover, BNT327 exhibited an acceptable tolerability profile, with a low discontinuation rate and no treatment-related deaths reported. All patients experienced at least one adverse event related to treatment with BNT327 and/or chemotherapy, with 86% of patients experiencing Grade 3 or higher treatment related adverse events (TRAEs). The most observed TRAEs were neutrophil count decrease, anemia, white blood cell count decrease and platelet count decrease.
A phase 2, single arm study, NCT05879068, assessing the efficacy and safety of BNT327 in combination with paclitaxel as second line (2L) treatment for SCLC who failed first line platinum-based chemotherapy with or without checkpoint inhibitors therapy. BNT327 combined with paclitaxel demonstrated encouraging efficacy in both immune-oncology (IO) -naive and IO treated patients. BNT327 exhibited an acceptable tolerability profile, with a low discontinuation rate and adverse events consistent with those expected from chemotherapy and in line with the expected mode of action of BNT327. 98.6% of patients experienced at least one adverse event. Among these, 78.6% of patients experienced Grade 3 or higher TRAEs such as neutrophil count decrease, white blood cell count decrease, anemia and platelet count decrease. Two grade 5 TRAEs occurred: pneumonitis and immune-mediated hepatitis.
These preliminary data of BNT327 in combination with chemotherapies on SCLC reveal promising outcomes, whether in 1L or 2L treatment. This bsAb exhibited an acceptable tolerability profile and adverse events consistent with those expected.
Patent family related to BNT327 clinical trial (WO2022/042719)
Biotheus has 11 patent families in bispecific antibody for oncology treatment (see the latest KnowMade’s report: Bispecific Antibody & Cancer) published between 2019 and 2024. This patent portfolio represents 193 documents with worldwide applications: 65 in China, 39 in Europe, 37 in Australia, 32 in Korea, 28 in Japan, 27 in the USA, etc. 88 % are pending applications and 9% are granted patents.
One of these patent families (WO2022/042719) describes an anti-VEGF / anti-PD-L1 bsAb. Published in 2022, it describes a molecule with two functional domains: one targeting VEGF to inhibit tumor angiogenesis and the other targeting PD-L1 to restore immune system activity (Figure 1).
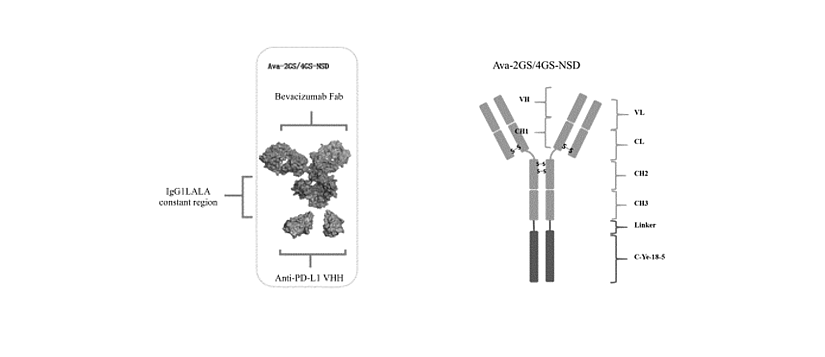
Figure 1: schematic diagram of the structure of Ava-2GS-NSD or Ava-4GS-NSD.
It is composed of 4 polypeptide chains (2 heavy chains ligated to C-Ye-18-5 respectively, and 2 light chains) in which both peptide chains #1 had the amino acid sequence set forth in SEQ ID NO: 1 or SEQ ID NO: 2, which contained the VH amino acid sequence (SEQ ID NO: 3) derived from the anti-VEGF antibody Bevacizumab, the C-terminal of the VH amino acid sequence was directly ligated to the CH amino acid sequence derived from human IgG1 (LALA mutation was introduced to reduce the Fc function, SEQ ID NO: 4), and the N-terminal of the anti-PD-L1 nanobody C-Ye-18-5 (SEQ ID NO: 5) was ligated to the C-terminal of the heavy chain through a flexible peptide of 11 amino acid residues (G4S)2G (SEQ ID NO: 6) (Ava-2GS-NSD) or 21 amino acid residues (G4S)4G (SEQ ID NO: 7) (Ava-4GS-NSD). Both peptide chains #2 had the amino acid sequence set forth in SEQ ID NO: 8, which contained the VL amino acid sequence (SEQ ID NO: 9) derived from the anti-VEGF antibody Bevacizumab, and the human κ light chain constant region (CL) amino acid sequence (SEQ ID NO: 10) ligated to the C-terminal of the VL amino acid sequence.
In examples, several experiments demonstrated that anti-VEGF / anti-PD-L1 bsAbs completely block the interaction between VEGF and VEGFR protein, activate T cells and have good thermal stability. In pharmacokinetic evaluation in rats, the half-life of molecule was about 320.6 hours. Then, in human colon cancer LOVO cells/NOG mice injected with human PBMC model, bsAbs could inhibit tumor growth in a dose-dependent manner (Figure 2). The effective dose was 4.8 mg/kg and no obvious toxicity to mice was observed at the 3 doses (1 mg/kg, 4.8 mg/kg and 24 mg/kg).
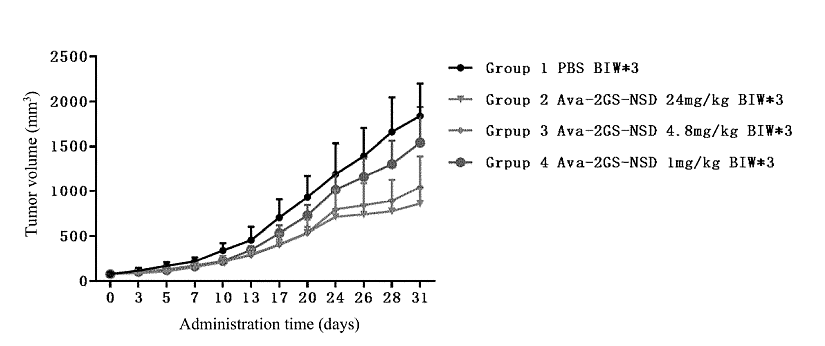
Figure 2: diagram of the effect of the bispecific antibody on the tumor volume in mice.
The results showed that, compared with the PBS group, Ava-2GS-NSD could inhibit tumor growth in a dose-dependent manner, and the tumor growth inhibition (TGI) values at 1 mg/kg, 4.8 mg/kg and 24 mg/kg doses were 16.7%, 44.9% and 55.6%, respectively. The tumor volume of the 4.8mg/kg and 24mg/kg groups was significantly different from that of the PBS group. The results of average tumor weight, tumor growth inhibition rate and tumor volume substantially showed similar trends.
Regarding legal status, the patent family WO2022/042719 includes pending applications worldwide. In the international patent application process, the written opinion of the International Search Authority (ISA) plays a crucial role by providing a preliminary analysis of the patentability of the invention. This assessment is based on the criteria of novelty, inventive step, and industrial applicability, allowing the applicant to anticipate potential objections during subsequent stages of intellectual property protection.
According to the ISA, the priority was deemed valid, but certain claims, such as claim 22, were not examined due to their surgical nature, which is excluded from international searches under PCT Rule 39.1. The opinion is based on prior art documents, notably patent CN109942712 (D1), which describes a similar bifunctional antibody, and a Genbank sequence (D2) related to the anti-VEGF antibody.
The analysis concludes that several claims (notably 1, 5-6, 10, 12-17, 20-21, 23-24, 27-33) lack novelty, as D1 already discloses all their technical features. However, claims 2-4, 7-9, 11, 18-19, and 25-26 are considered novel. Nonetheless, some of these lack inventive steps, as their features are deemed obvious choices for a skilled person in the field, particularly due to the use of known sequences or standard methods. Claim 3 stands out for its inventive character, as the particular structure of the antibody is neither disclosed nor suggested in the prior art.
The opinion also highlights a lack of experimental support for claims 1 to 4 regarding the bispecific antibody’s ability to simultaneously bind VEGF and PD-L1. The absence of details on the CDR sequences of the functional domains raises doubts about the specificity and binding affinity of the antibody. Therefore, the authority considers these claims insufficiently supported by the description, in violation of Article 6 of the PCT. Additional experimental data could thus strengthen the application by demonstrating the effectiveness of the claimed dual-specific binding.
BNT327: An Innovative Therapy with High Potential to Redefine Solid Tumor Treatment
In conclusion, BNT327 represents a new generation of cancer therapy, simultaneously targeting the PD-L1 and VEGF pathways in an innovative approach that could surpass Keytruda as the standard of care in oncology. Although challenges remain in securing broad protection for some claims, the positive results of pharmacokinetic and clinical data indicate that this therapy has the potential to change the management of solid tumors.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About the author
Fabienne Massa, PhD., works for Knowmade in the field of Biotechnology and Life Sciences. She holds a PhD in Molecular and Cellular Biology from the IPMC (Sophia Antipolis, France). She also holds a Master of Business Management from IAE (Nice, France) and she previously worked in the pharmaceutical industry.
About KnowMade
KnowMade is a technology intelligence and IP strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patent information and scientific literature into actionable insights, providing high added value reports for decision makers working in R&D, innovation strategy, intellectual property, and marketing. Our experts provide prior art search, patent landscape analysis, freedom-to-operate analysis, IP due diligence, and monitoring services.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Sensors, Semiconductor Packaging, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
