SOPHIA ANTIPOLIS, France – February 01, 2021 | After three decades of research, mRNA-based vaccines have proven their safety and efficacy. They have finally reached the market, holding the promise to revolutionize the field. In December 2020, the FDA and EMA granted historic authorization to the first mRNA vaccine in the United States and in Europe. mRNA vaccines against COVID-19 have demonstrated efficacy rates of 94% (mRNA-1273 Moderna) and 95% (BNT162b2 BioNTech) in large-scale trials, respectively.
Other companies developing COVID-19 mRNA vaccines could also obtain marketing authorization soon, such as CureVac (CVnCoV), Arcturus (ARCT-021), Imperial College London (LNP-nCoVsaRNA), Chulalongkorn University (ChulaCov19) and HDT Bio/Gennova (HDT-301/HGCO19). In this evolving context, it is crucial to understand the intellectual property (IP) position and strategy of these different players.
Based on the RNA vaccine patent landscape published by Knowmade in January 2021, CureVac, BioNTech and Moderna emerged as the top players in the mRNA vaccine field. However, although CureVac’s COVID-19 RNA vaccine candidate has not yet been authorized, the company is a pioneer in this field, holding the largest patent portfolio. Its first patent family identified was published in 2001, and its patent portfolio comprises over 100 patent families. Moreover, its patent portfolio comprises the highest number of ‘alive’ patents (370 granted and 400 pending patents) with a large geographical coverage, giving CureVac the strongest IP position in mRNA vaccines. Its patent portfolio is also the one that is primarily targeted by competitors (including BioNTech and Moderna) with over 10 oppositions pending before the European Patent Office (EPO). These challenged patents could potentially hamper competitors’ freedom to operate. For example, these attacked patents disclosed:
• Optimized mRNA (chemical modifications: EP1797886; modified 5’cap structure: EP1685844; optimized G/C content: EP1392341, EP2680881, EP2680880);
• Injection formulation for mRNA (EP3153179);
• mRNA sequence coding for an antibody for infectious disease and cancer vaccines (EP2101823);
• cancer vaccine: (mRNA coding for a checkpoint modulator PD-1 pathway inhibitor (EP3292873, EP2958588, EP3173092, EP3326641), or a tumor necrosis factor receptor OX40 (EP3116535).
CureVac recently disclosed that its COVID-19 vaccine candidate (CVnCoV) is using unmodified mRNA allowing improved induction of the body’s viral defenses, including interferon type 1. According to preliminary results, its vaccine candidate exhibits up to 24 hours’ stability at room temperature and at least 3 months at 2-8°C. Thus CureVac’s mRNA technology applied to its vaccine candidate allows it to outperform the stability of its main competitors, i.e. Moderna (stable at -20°C) and BioNTech (stable at -70°C). Moreover, the EMA has authorized vaccines by Moderna and BioNTech for 100 µg and 30 µg doses, while preclinical data from CVnCoV supports protective efficacy at low doses (12 µg). Considering its strong IP position, stability and efficacy data at low doses, CVnCoV may therefore have strong advantages over its competitors.
The exact technologies leading to these differences in stability and efficacy were not disclosed. Focusing on CureVac’s mRNA technology, here are some patents identified in the RNA vaccine patent landscape disclosing technologies to enhance mRNA stability and translation efficiency:
• optimized 5’cap (WO2019/175356),
• 5’UTR (WO2013/143699, WO2013/143700),
• G/C content (EP1604688),
• poly(A) tail (WO201691391) and
• 5’ and/or 3’ UTR (WO2016/107877, WO2017/036580)
For example, WO2019/175356 disclosed optimized 5’caps that have been shown to maintain high protein expression levels for at least 72 hours. As shown in the figure below, the presence of a cap l structure in RNA results in much higher expression levels of mEPO compared to RNA with a cap O structure, particularly 24 hours after injection.
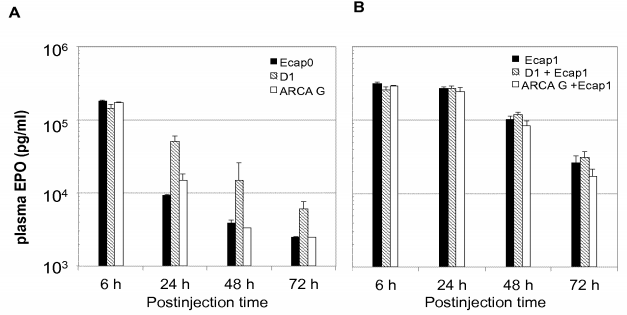
In vivo translation of murine erythropoietin (mEPO) RNAs modified with different 5 ‘-cap analogs having a capO structure (A) or a capl structure (B). ARCA G: RNA co-transcriptionally capped with ARCA G; Dl : RNA co-transcriptionally capped with the Dl diastereomer of beta-S-ARCA; EcapO: RNA enzymatically capped providing a capO structure; ARCA G+Ecapl: RNA co- transcriptionally capped with ARCA G, then enzymatically capped using vaccinia capping enzyme and vaccinia methyltransferase which provide a capl structure; Dl+Ecapl : RNA co-transcriptionally capped with the Dl diastereomer of beta-S-ARCA, then enzymatically capped using vaccinia capping enzyme and vaccinia methyltransferase which provide a capl structure; Ecapl : RNA enzymatically capped providing a capl structure. mEPO mRNA (3 pg) containing l-methylpseudouridine (ml T) were formulated in TransIT® and injected i.p. into mice.
Delivery system is another essential compound for stability and protection of the RNA molecule. However, it is unlikely that the difference observed is related to the delivery system, as both CureVac and BioNTech rely on Acuitas’ lipid nanoparticle (LNP) technology.
The patent situation over its LNP delivery system is not clear yet and, like BioNTech, CureVac may have to get a license from Genevant to access Arbutus Biopharma key LNP technology (e.g. US8058069). Indeed, following a litigation settlement in 2017, Acuitas’ rights to sublicense Arbutus LNP Technology (e.g. US8058069) to any third party was terminated. Then in 2018, Arbutus and Roivant launched Genevant Sciences, a jointly-owned company focused on the discovery, development and commercialization of a broad range of RNA-based therapeutics enabled by Arbutus’ proprietary LNP.
Beyond a COVID-19 vaccine, RNA technology holds promise for vaccines that can efficiently trigger an immune response against cancer and challenging viruses that conventional vaccines have failed to address (e.g. HIV-1, herpes simplex virus, respiratory syncytial virus). Contrary to conventional vaccines, mRNA vaccines have shown promising results for cancer therapy, triggering antigen-specific T cell responses. The technology also allows for personalized immunotherapy, or cancer neoantigen vaccines, by matching the genetic profile of each person’s cancer and inducing an immune response against the mutated part of the tumors.
CureVac’s pipeline includes vaccines for infectious diseases and cancer immunotherapies that are likely to be covered, at least partially, by the following patent families: Rabies (WO2015/024665), Lassa (WO2018/115525, WO2020/002525), yellow fever (WO2019/193183, WO2019/115635), Respirational syncytial virus (WO2015/024668, WO2019/202035), Rotavirus (WO2020/254535, WO2017/081110), malaria (WO2020/128031), universal influenza (WO2017/191258, WO2019/092153), cutaneous melanoma/adenoid cystic carcinoma/squamous cell cancer of skin/head and neck (CV8102; WO2018/033254) and non-small cell lung cancer (BI1361849/CV9202; WO2009/046738 filed jointly with Boehringer; WO2015/024666).
Over the years, CureVac has taken part in many collaborations for the development of different projects. In the field of cancer immunotherapy, CureVac has been collaborating with the Ludwig Cancer Research Institute since 2013. In August 2014, CureVac granted an exclusive license for the development and commercialization of CureVac investigational therapeutic mRNA lung cancer vaccine BI1361849, and products containing such a vaccine, for all uses related to cancer in humans, to Boehringer Ingelheim. A jointly filed patent family, WO2018/167320, was identified, claiming an mRNA vaccine coding for PD-1 and LAG-3 pathway inhibitors for the treatment of cancer, including lung cancer. Results show that RNA vaccination in combination with anti-PD-1 and anti-LAG-3 treatment decreases tumor growth in an E.G7-OVA tumor model (figure below).
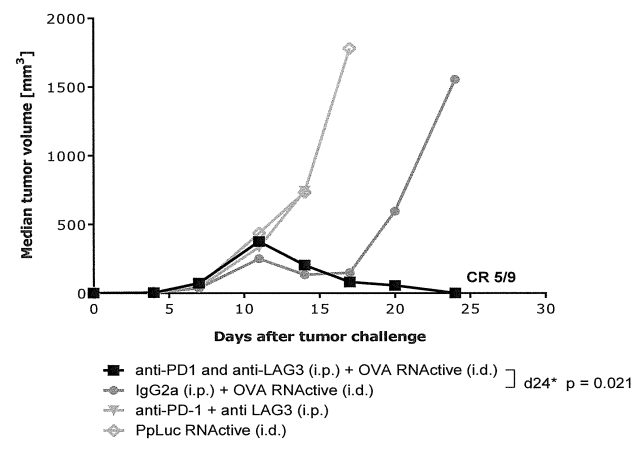
C57BL 6 mice (n > 9 per group) were challenged subcutaneously on the right flank with 3 x 105 syngeneic E.G7-OVA lymphoma cells (ovalbumin expressing EL4 lymphoma cell line). Four days after tumor cell inoculation mice were vaccinated intradermally with OVA-encoding RNA (“RNActive”) and treated intraperitoneal^ with 200 pg anti-PD-1 (BioXcell) and 200 pg anti-LAG-3 antibody (BioXcell) twice a week for three to four weeks. Mice treated with unspecific RNA vaccination served as controls.
In 2011, CureVac entered into collaboration and a license option agreement with Sanofi Pasteur for several pre-defined pathogens. CureVac then executed an exclusive license agreement in 2014 to develop and commercialize an mRNA-based vaccine against an undisclosed pathogen. As part of their collaboration, two jointly filed patent families directed at Zika and Flavivirus vaccines were identified (WO2017/140905 and WO2019/115635). Results of WO2017/140905 show that a concentration-dependent immune response was triggered by vaccination with tested ZIKV mRNA vaccines in a hamster.
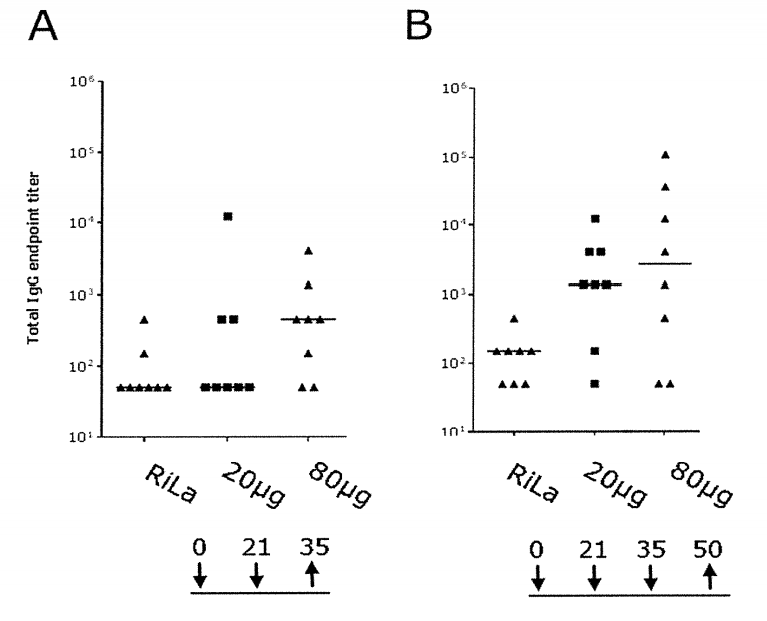
The injected ZIKV prME mRNA vaccine induced binding antibodies against the E protein in vaccinated hamster. After the third vaccination, stable and high IgG antibody titers were observed for both concentrations, showing that a concentration dependent immune response was triggered by vaccination with tested ZIKV mRNA vaccines in hamster.
In 2015, the Bill & Melinda Gates Foundation invested in CureVac to support the development and production of numerous vaccines against infectious diseases that disproportionately affect people in the world’s poorest countries (e.g. Rota, malaria, universal influenza). In 2017, CureVac entered into a license agreement with Eli Lilly for the development and commercialization of up to five potential cancer vaccine products. In June 2020, however, Eli Lilly announced early termination of the agreement. In 2018, CureVac entered into collaboration with Arcturus to access its lipid-mediated delivery system, LUNAR™, to develop mRNA product candidates, primarily targeting ornithine transcarbamylase (OTC) deficiency. In July 2020, GSK and CureVac entered into collaboration to develop up to five mRNA-based vaccines against infectious diseases and monoclonal antibodies targeting infectious disease pathogens (COVID-19 excluded). In February 2019, the Coalition for Epidemic Preparedness Innovations (CEPI) invested in CureVac to support and accelerate the development of vaccines against emerging infectious diseases, such as COVID-19, Lassa and yellow fever. In January 2021, CureVac struck a deal with Bayer to accelerate the development and supply of its COVID-19 vaccine candidate.
In addition to the development of a Covid vaccine, these different collaborations therefore underline CureVac’s presence in numerous different RNA vaccine projects, demonstrating the company’s strong know-how developed over the years.
In conclusion, the pandemic has accelerated the emergence of the RNA technology that finally succeeded in reaching the market. Substantial cash flow from sales of the COVID-19 vaccine, and expansion of production capacity, should benefit millions of people by making new infectious-disease and cancer vaccines available much faster. On the IP side, a number of infringement actions could arise after the pandemic, as products reach this promising market. In this context, CureVac should take advantage of its leading IP position by asserting its patents against competitors through licenses and infringement suits. Given the potential markets concerned by RNA vaccines and their advantages over other technologies, the income generated by these actions could be very significant.
Identify all key information about healthcare innovation with our patent landscape reports.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About our analysts
Matthieu Besse, PhD. Matthieu works for Knowmade as a Patent Attorney and Patent Analyst in the field of Biotechnology and Life Sciences. He holds a PhD in molecular from UCD (University College of Dublin, Ireland), and he is a part-qualified European patent attorney.
About Knowmade
Knowmade is a Technology Intelligence and IP Strategy consulting company specialized in analysis of patents and scientific information. The company helps innovative companies and R&D organizations to understand their competitive landscape, follow technology trends, and find out opportunities and threats in terms of technology and patents.
Knowmade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patents and scientific information into business-oriented report for decision makers working in R&D, Innovation Strategy, Intellectual Property, and Marketing. Our experts provide prior art search, patent landscape analysis, scientific literature analysis, patent valuation, IP due diligence and freedom-to-operate analysis. In parallel the company proposes litigation/licensing support, technology scouting and IP/technology watch service.
Knowmade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Solid-State Sensors/Actuators, Semiconductor Manufacturing, Packaging & Assembly, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
