SOPHIA ANTIPOLIS, France – January 19, 2026 │ The mRNA therapeutics landscape maintained strong momentum in Q4 2025 with 200 new patent applications and a record 125 granted patents across key jurisdictions (US, EP, JP, KR). This quarter highlights the expanding global reach of RNA-based technologies and shifts in strategic IP positioning. Therapeutic mRNA patent monitor reveals key insights to follow RNA-based therapeutics.
New Applications: PopVax Joins the Ranks of Key Innovators
Among the top filers this quarter, BioNTech (10) and University of Pennsylvania (9) continue to lead mRNA innovation. However, the emergence of PopVax (5)—an Indian biotechnology company—marks a key development. With a growing pipeline and IP portfolio in mRNA-based therapeutics, PopVax reflects India’s increasing role in next-generation biologics. [Read our dedicated Insight on PopVax’s strategy here.]
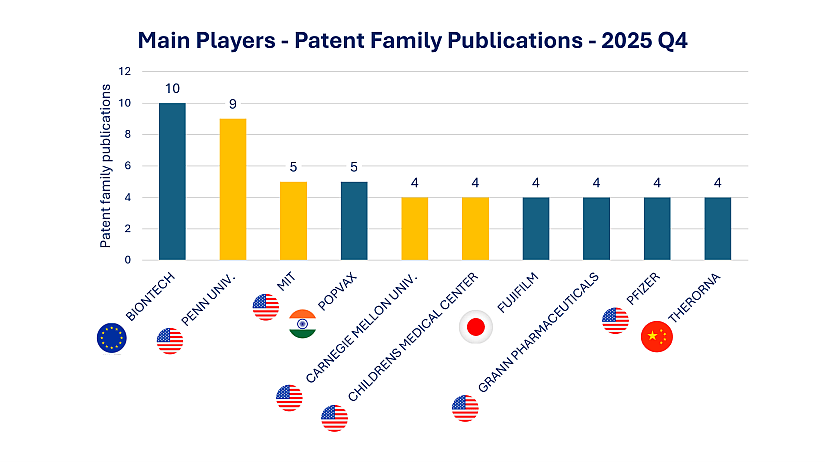
Figure 1: Q4 2025 Patent Filings by Key mRNA Therapeutics Innovators
In yellow, academic players; in blue industrial players
Granted Patents: BioNTech Expands via CureVac’s Legacy Portfolio
A total of 125 new mRNA patents were granted in Q4 2025, reflecting growing enforceability in the space. Sanofi secured 19 grants—2 directly filed and 17 inherited through its acquisition of Translate Bio, confirming the long-term integration of this platform into Sanofi’s IP strategy.
Moderna received 12 new patents, reaffirming its leadership in delivery and formulation technologies.
The integration of CureVac’s patent assets into BioNTech’s portfolio was a defining feature of this quarter. In December 2025, BioNTech completed the acquisition of CureVac N.V. following an exchange offer where approximately 86.75% of CureVac shares were tendered, enabling BioNTech to augment its capabilities in mRNA design, delivery formulations, and manufacturing (source). This strategic acquisition not only bolsters BioNTech’s R&D breadth but also expands its IP estate significantly: the eight newly granted patents originally filed by CureVac add depth to BioNTech’s existing portfolio, strengthening the company’s position in competitive mRNA technology domains.
Additional key grantees include Acuitas (7) and Nutcracker (4), both continuing to advance IP in lipid nanoparticle technologies.
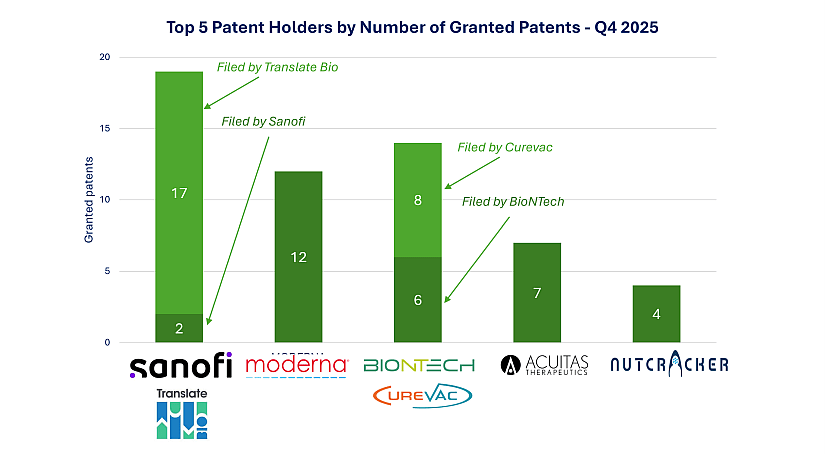
Figure 2: Top 5 Patent Holders by Number of Granted Patents – Q4 2025
Why This Matters for the mRNA Landscape
With 200 new patent publications and 125 granted patents recorded in Q4 2025 alone, the therapeutic mRNA sector continues to show innovation and growing global competition. Q4 2025 highlights several critical trends shaping the mRNA IP ecosystem:
- Diversification of innovation sources, with emerging players like PopVax earning notable publication counts.
- The strategic role of acquisitions—BioNTech’s integration of CureVac patents underscores how M&A can reshape competitive IP positioning.
- Academic contributions remain vital, accounting for a meaningful share of high-impact filings.
- Regional shifts in filing patterns may influence future collaboration and investment strategies.
KnowMade’s Therapeutic mRNA Patent Monitor provides in‑depth, actionable intelligence on the evolving mRNA intellectual property landscape. By tracking key players, emerging technologies, and global filing trends, the monitor highlights major dynamics.
Through comprehensive and continuously updated patent analysis across major jurisdictions, KnowMade supports organizations in identifying collaboration or licensing opportunities, strengthening the protection of their innovations, and confidently navigating complex IP challenges.
Whether monitoring competitors, guiding R&D and innovation strategies, or entering the therapeutic mRNA field, KnowMade’s expert‑driven insights deliver the clarity and depth required to make informed decisions and remain competitive in a rapidly evolving market.
Contact us to learn how KnowMade’s monitoring can support your innovation and IP strategy.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About KnowMade
KnowMade is a technology intelligence and IP strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
