SOPHIA ANTIPOLIS, France – October 24, 2025 │ Hansoh Pharmaceutical has signed a license agreement with Roche to develop HS-20110, a CDH17-targeting antibody-drug conjugate (ADC) utilizing a clinically validated topoisomerase inhibitor payload. Hansoh Pharma gives Roche an exclusive worldwide license (excluding the Chinese Mainland, Hong Kong, Macau and Taiwan) to advance the clinical development and commercialization of HS-20110. Roche will pay $80 million upfront in the deal. According to the Chinese collaborator, they are entitled to development, regulatory, and commercialization milestones, but they did not specify the amount.
Innovating for the future: Hansoh Pharma’s strategic focus on targeted therapies and ADCs
Founded in 1995 and headquartered in China, Hansoh Pharmaceutical Group, also known as Hansoh Pharma, is one of China’s leading innovation-driven pharmaceutical companies. Listed on the Hong Kong Stock Exchange since 2019, the company develops, manufactures, and commercializes both innovative and generic medicines across major therapeutic areas, including oncology, central nervous system disorders, infectious diseases, metabolism, and autoimmune conditions. Hansoh operates as a fully integrated R&D-driven organization, supported by more than 700 patent families and four research centers located in China and the United States.
In the oncology field, Hansoh Pharma has gained recognition for developing targeted therapies and antibody-based biologics. Its flagship product, Aumolertinib (Ameile), a third-generation EGFR inhibitor, is approved in China for the treatment of non-small cell lung cancer and exemplifies the growing strength of domestic clinical innovation. The company is also advancing several immuno-oncology candidates, including monoclonal antibodies and antibody-drug conjugates, aiming to meet global biopharmaceutical standards. Hansoh’s ADC platform, integrating antibody engineering, novel tumor target discovery, and optimized cytotoxic payload design, represents a strategic pillar of its oncology pipeline.
HS-20110: a next-generation ADC targeting cadherin 17 in solid tumors
HS-20110 is an ADC targeting cadherin-17 (CDH17), a cell adhesion molecule that maintains the integrity of tissues. It is a membranous cell adhesion protein predominantly expressed in intestinal epithelial cells. The ADC is linked to topoisomerase inhibitor payload. Topoisomerases are enzymes that can alter DNA topology in eukaryotic cells.
Safety and tolerability of HS-20110 in advanced solid malignant tumors
On December 16, 2024, HS-20110 for injection obtained the Clinical Trial Approval issued by the National Medical Products Administration (NMPA) of China, which is intended to be investigated in clinical trials for advanced solid tumor.
The ongoing first-in-human study NCT06892379 is an open-label, multicenter phase I dose-escalation/expansion trial of HS-20110, in adults with pathologically confirmed advanced solid tumors. Sponsored by Hansoh Biomedical R&D, the trial aims to characterize safety, tolerability, pharmacokinetics, and preliminary antitumor activity, with an estimated enrollment of 475 patients. The study started on February 26, 2025, and is still recruiting. It lists an estimated primary completion in December 2026 and study completion in September 2027. U.S. sites include BRCR Medical Center in Florida and The University of Texas MD Anderson Cancer Center, underscoring the program’s global footprint.
Building a versatile ADC platform: Hansoh’s strategic patents on anti-CDH17 conjugates
Hansoh Pharma owned 780 patent families whose 2 on ADC area (7 documents – Europe, Australia, New Zealand, China, Taiwan, Mexico, Brazil), filed in 2024 (pending applications).
The ADC related in WO2024/199337 patent family is an anti-CDH17 antibody conjugated to a toxin drug by a linker for cancer treatment. In claims, many drugs are described: tubulin inhibitors (e.g., auristatin and maytansine analogues such as MMAE, MMAF, DM1, DM4) and topoisomerases inhibitors (e.g., camptothecin derivatives such as SN-38, exatecan, Dxd). In in vivo studies disclosed in this patent application, it is not the ADC HS-20110, conjugated to topoisomerase inhibitor, which is used but an ADC with MMAE drug. Studies were performed in female BALB/c nude mice, engrafted subcutaneously with AsPC1 cells (pancreatic cancer) or with GP2d cells (colorectal cancer). The mice were treated with ADCs (6 mg/kg) intravenously Q4D x 3. Tumor growth in mice treated with ADC-02, ADC-05, ADC-06, ADC-07, and ADC-10 was inhibited compared to the vehicle/PBS group (figure 1). Among them, the tumor growth of ADC-02, ADC-05, ADC-07, and ADC-10 was inhibited compared to the ADC-11 positive control group. The tumor volumes in ADC-11 group increased after around 30 days.
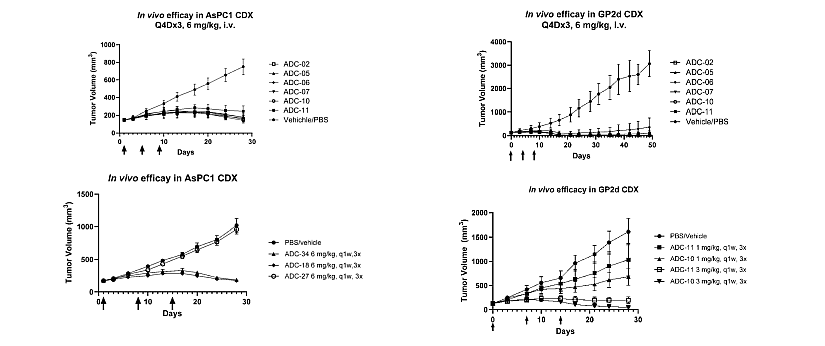
Figure 1: Tumor-Inhibitory Experiment of ADCs Towards CDH17 Positive Cancer Cell Nude Mouse Subcutaneous Transplantation Tumor Model. a) Anti-CDH17 ADCs inhibit tumor growth in AsPC1 tumor-bearing mice. B) Anti-CDH17 ADCs inhibit tumor growth in GP2d tumor-bearing mice.
The second patent family, WO2025/087264, extends and strengthens Hansoh’s intellectual property protection around its anti-CDH17 ADC. Claims are focused on the ADC formula; no drug is described. In the description, topoisomerase inhibitors and tubulin inhibitors are advanced such as camptothecin derivatives such as SN-38, exatecan, Dxd and auristatin / maytansine analogues such as MMAE, MMAF, DM1, DM4. This strategic continuation demonstrates Hansoh’s intent to protect a versatile ADC platform adaptable to multiple payload classes, ensuring freedom to operate for future developments beyond HS-20110. From a competitive intelligence perspective, this second patent family underlines Hansoh’s effort to build a broad IP perimeter around CDH17 as a therapeutic target in gastrointestinal and other solid tumors, while aligning its claims with the molecule currently in clinical evaluation.
Hansoh and Roche strengthen IP and global ties in the ADC Landscape
Hansoh Pharmaceutical’s recent patent filings on CDH17-targeting ADCs illustrate a clear intent to secure intellectual property around its innovative antibody-drug conjugate platform. By covering both topoisomerase and tubulin inhibitor payload classes, Hansoh aims to establish a broad technological perimeter and reinforce its competitive position in oncology. The deal with Roche builds on this foundation, positioning Hansoh as a key innovator in the ADC field. For Roche, already a major player in ADCs, this collaboration follows its 2024 partnership with MediLink Therapeutics on a c-Met–directed ADC, reinforcing its investment in Chinese innovation. Hansoh’s recent alliances, including with GSK for rezetecan, reflect growing global confidence in China’s biopharma capabilities. In a broader context, Western biopharma firms invested over $48.5 billion in Chinese partnerships in the first half of 2025, underscoring the country’s emergence as a crucial hub for next-generation oncology assets.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About the author
Fabienne Massa, PhD., works for KnowMade in the field of Biotechnology and Life Sciences. She holds a PhD in Molecular and Cellular Biology from the IPMC (Sophia Antipolis, France). She also holds a Master of Business Management from IAE (Nice, France) and she previously worked in the pharmaceutical industry.
About KnowMade
KnowMade is a technology intelligence and IP strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patent information and scientific literature into actionable insights, providing high added value reports for decision makers working in R&D, innovation strategy, intellectual property, and marketing. Our experts provide prior art search, patent landscape analysis, freedom-to-operate analysis, IP due diligence, and monitoring services.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Sensors, Semiconductor Packaging, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
