SOPHIA ANTIPOLIS, France – January 30, 2025 │ Roche’s new $570M near‑term licensing deal for MediLink’s YL201 underscores the strategic premium placed on differentiated ADC assets—and on the IP required to defend them.
Roche announced an exclusive licensing agreement with China’s MediLink Therapeutics for YL201, an investigational antibody–drug conjugate (ADC) targeting B7-H3 (CD276). Roche will pay up to $570 million in upfront and near‑term milestone payments and will be responsible for global development, manufacturing, and commercialization outside mainland China, Hong Kong and Macau. The deal also includes additional development, regulatory and commercial milestones, plus tiered royalties (amounts not publicly disclosed).
Roche–MediLink: a $570M near-term licensing agreement centered on YL201
From a strategic perspective, this transaction is less about a single asset and more about the underlying trend: large pharma is increasingly securing late‑stage or fast‑advancing ADC programs through cross‑border licensing, while technology differentiation (payload/linker design, conjugation chemistry, and stability/activation profiles) and freedom‑to‑operate become central to valuation.
MediLink Therapeutics is an innovative drug development company founded on 2020, focused on antibody–drug conjugates and related technologies, with operations centered in Suzhou, China. The company positions its proprietary TMALIN® (Tumor Microenvironment Activable LINker-payload) platform as a core differentiator, designed to generate homogeneous ADCs with a high drug–antibody ratio (DAR) and an improved therapeutic window, notably by combining high plasma stability with controlled payload release (including tumor-microenvironment activation).
Roche is a Basel-based healthcare company founded on 1896 in Basel, Switzerland, operating globally across pharmaceuticals (and, more broadly as a group, diagnostics). In oncology, Roche emphasizes a long-standing commitment (50+ years) to developing cancer medicines and highlights continued investment in personalised approaches, including immunotherapy, supported by an active pipeline and clinical development programs across major tumor areas (e.g., lung and breast cancer).
YL201: a B7‑H3‑targeting ADC with
a camptothecin/Topoisomerase‑I inhibitor payload
YL201 is a human anti‑B7‑H3 monoclonal antibody conjugated to camptothecin, a Topoisomerase-1 inhibitor, via a protease‑cleavable linker, with a DAR of 8.
B7-H3 is an immune checkpoint glycoprotein. It promotes immune tumor evasion and contributes to cancer progression, making it an attractive target for ADC development. Its overexpression is frequently associated with a poor prognosis.
Clinical readouts available: Phase 1/1b program
A Nature Medicine publication give some clinical results about 2 studies, NCT05434234 and NCT06057922, titled “A B7H3-targeting antibody–drug conjugate in advanced solid tumors: a phase 1/1b trial” (link). A total of 312 patients were enrolled across multiple tumor types, including extensive-stage small cell lung cancer, nasopharyngeal carcinoma, non-small cell lung cancer, esophageal squamous cell carcinoma and other solid tumors.
The NCT05434234 study (2022) is a phase 1, multicenter, nonrandomized, open-label, first-in-human study of YL201 conducted in China and the United States. The study includes 2 parts: a dose escalation part (Part 1) followed by a dose expansion part (Part 2). Part 1 estimates the maximum tolerated dose (MTD) in dose escalation cohorts of patients with advanced solid tumors unresponsive to currently available therapies or for whom no standard therapy is available. Part 2 includes patients with selected advanced solid tumor types enrolled at the MTD, to better define the safety profile and evaluate the efficacy of YL201.
The NCT06057922 study (2023) is a multicenter, open-label, phase 1/2 study to evaluate the safety, efficacy, and pharmacokinetics of YL201 in patients with selected advanced solid tumors. The study includes 2 parts: Phase 1 dose expansion stage (Part 1) followed by a Phase 2 stage with expanded sample size (Part 2). Part 1 estimates the Recommended Phase II Dose (RP2D) in dose expansion cohorts of patients with not limited to non-small cell lung cancer, small cell lung cancer, nasopharyngeal carcinoma, esophageal squamous cell carcinoma, metastatic castration-resistant prostate cancer, head and neck squamous cell carcinoma, sarcoma, ductal adenocarcinoma of pancreas, hepatocellular carcinoma, biliary tract cancer, etc. Part 2 includes patients with selected advanced solid tumor types enrolled at the RP2D to further assess the efficacy and safety of YL201.
Preliminary data revealed by MediLink:
- Doses: The maximum tolerated dose (MTD) was determined to be 2.8 mg kg−1, and the recommended expansion dose was selected as 2.0 mg kg−1 and 2.4 mg kg−1 every 3 weeks.
- Overall response rate (ORR): Encouraging anti-tumor activity was observed, particularly in patients with ES-SCLC (ORR, 63.9%), nasopharyngeal carcinoma (ORR, 48.6%), lung adenocarcinoma (ORR, 28.6%) and lymphoepithelioma-like carcinoma (ORR, 54.2%). No significant correlation between B7H3 membrane expression and the ORR was found.
- Safety Results: The most common grade 3 or higher treatment-related adverse events included neutropenia (31.7%), leukopenia (29.5%) and anemia (25.0%). Only 4 cases of interstitial lung disease (1.3%) and 1 case of infusion reactions (0.3%) were observed.
YL201 demonstrated an acceptable safety profile and a promising efficacy in heavily pretreated patients with advanced solid tumors.
Other studies with no clinical results published:
| NCT Number | Start Date | Phase | Condition | Intervention | Sponsor | Study Title |
| NCT06241846 | 2024 | PHASE2 | Metastatic Castration-resistant Prostate Cancer | YL201 | MediLink | A Phase II Study to Evaluate the Safety, Efficacy, and Pharmacokinetics of YL201 in Patients With mCRPC |
| NCT06394414 | 2024 | PHASE1 | Advanced Solid Tumors | YL201 | MediLink | A Phase 1 Study to Evaluate the Safety, Efficacy, and Pharmacokinetics of YL201 in Advanced Solid Tumors |
| NCT06612151 | 2024 | PHASE3 | Small Cell Lung Cancer | YL201|topotecan hydrochloride | MediLink | A Phase III Study of YL201 in Relapsed Small Cell Lung Cancer |
| NCT06629597 | 2024 | PHASE3 | Nasopharyngeal Carcinoma | YL201|Docetaxel|Capecitabine|Gemcitabine | MediLink | A Phase III Study of YL201 in Recurrent or Metastatic Nasopharyngeal Carcinoma |
| NCT06898957 | 2025 | PHASE1 | Small Cell Lung Cancer | YL201|Tarlatamab|Atezolizumab|Durvalumab | MediLink Amgen |
Study of Tarlatamab in Combination With YL201 With or Without Anti-programmed Death Ligand 1 (PD-L1) in Participants With Extensive Stage (ES) SCLC |
| NCT07208773 | 2025 | PHASE1|2 | Advanced Solid Tumors|Small Cell Lung Cancer|NSCLC | YL201|Ivonescimab | MediLink Akesobio |
A Study of YL201 and Ivonescimab (AK112) in Advanced Solid Tumors |
| NCT07258979 | 2025 | PHASE1|2 | Recurrent or Metastatic Nasopharyngeal Carcinoma | YL201|Toripalimab|Cisplatin | MediLink | A Study of YL201 in Combination With Toripalimab and With or Without Cisplatin in Nasopharyngeal Carcinoma. |
Intellectual Property Supporting YL201 Development
MediLink Therapeutics owns 16 patent families (124 documents), filed between 2022 and 2024. These patent families have been identified in KnowMade’s report Cancer & Antibody Conjugates Patent Landscape Analysis 2025. The company has a strong worldwide IP strategy, with patent applications in Europe, the US and Asia. All its patents are currently alive (pending or granted patents), reflecting that its R&D efforts are still on going to improve the current technologies involving cancer therapies. The strength of its patent portfolio is expected to increase in the coming years, given that the vast majority of its active patents are currently pending.
There are 8 patent families (51 documents), filed between 2022 and 2024, which relates B7H3 ADC (claims and/or examples) but only 2 really described ADC against protein B7-homologue 3 (B7H3). For these 8 patent families, currently, the company has a national strategy with patent families published mainly in China (18). There are no patent family published in Europe and only 6 in the US
The ADC described in the patent family WO2025/061110 is an anti-B7H3 antibody drug conjugate which is used in the preparation of a drug for the treatment of nasopharyngeal carcinoma and/or pulmonary lymphoepithelioma-like carcinoma.
The phase 1, multicenter, non-randomized, open-label and first human study is performed to evaluate the safety, tolerability, pharmacokinetics and efficacy of anti-B7H3 ADC. This clinical trial was performed for treating nasopharyngeal carcinoma and pulmonary lymphoepithelioma-like carcinoma.
Preliminary clinical data are detailed for 5 men with nasopharyngeal carcinoma:
- After 18 weeks of treatment, #1: Tumor lesions located in the inferior lobe of the right lung have shrunk from 25mm to 5 mm, 80% (figure below).
- After 12 weeks of treatment:
- #2: the cervical tumor lesion was reduced from 15mm to 5 mm, a reduction of 66.7%.
- #3: the liver S7 tumor lesions were reduced from 44mm to 28mm, which was reduced by 36.4%; the liver S2 tumor lesions were reduced from 23mm to 16mm, which was reduced by 30.4%.
- #4: the right middle lung tumor lesion was reduced from 13mm to 7mm, 46.2%; left upper lung tumor lesion from 13mm to 9mm, reduced by 30.8%.
- After 6 weeks of treatment, #5: tumor lesions located in the middle lobe of the right lung were reduced from 15mm to 9 mm, 40%.
Preliminary clinical data are detailed for 3 women with pulmonary lymphoepithelioma-like carcinoma:
- After 24 weeks of treatment, #6: the tumor lesions located in the middle lobe of the right lung, the neck and the S7 segment of the liver are reduced from 23mm to 19mm, which is reduced by 17.4%; the tumor lesions in the neck are reduced from 19mm to 12mm, which is reduced by 36.8%; and the tumor lesions in the S7 segment of the liver are reduced from 16mm to 8mm, which is reduced by 50%.
- After 6 weeks of treatment:
- #7: cervical tumors were reduced from 15mm to 6 mm by 60%, and subpleural tumors were reduced from 16mm to 15mm by 6.3%.
- #8: left inferior lung lobe tumor lesion was reduced from 11mm to 9mm, 18.2%; left superior lobe tumor lesion was reduced from 13mm to 7mm, 46.2%; collarbone tumor lesion was reduced from 15mm to 11mm, 26.7%.
The anti-B7H3 drug-antibody conjugate targets the tumor and decreases tumor size in patients with solid cancer.
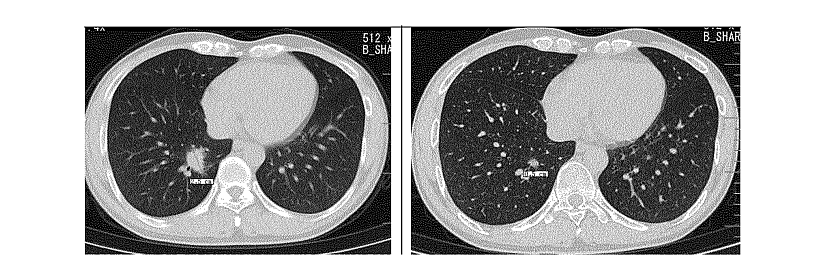
Figure: Inferior lobe of right lung in patient #1. A) baseline; B/ After 18 weeks of treatment with anti-B7H3 ADC.
The patent family WO2025/103379 describes a method of treating cancer with the anti-B7H3 ADC in combination with anti-PD-1 antibodies and a chemotherapeutic agent, selected from a platinum class of drugs. The anti-PD-1 antibody is administered to a subject in a uniform dose ranging from about 100mg to about 600mg or in a dose of 1-10mg/kg. .
A phase 1 clinical trial (NCT06394414) was performed for treating patients with advanced solid tumors. This multicenter and open clinical trial is to assess the safety, efficacy and pharmacokinetics of the anti-B7H3 ADC in combination with or without serplulimab (anti-PD-1 antibody – HETRONIFLY®).
Preliminary clinical data are detailed for 8 patients with multiple advanced solid tumors:
- After 18 weeks of treatment:
- #1: the lower lobe lesions of the right lung were reduced from 30mm to 11mm, which was reduced by 63.3%, and the subluminal lymph nodes lesions were reduced from 23mm to 11mm, which was reduced by 52.2%. The overall best efficacy assessment achieved confirmed partial response (PR).
- #2: the left lower lobe lesions from 34mm to 28mm, reduced by 17.6%, the right hilar lymph nodes from 22mm to 18mm, reduced by 9.1%, the left hilar lymph nodes from 20mm to 16mm, reduced by 20%. The overall best efficacy was assessed by stable disease (SD).
- After 12 weeks of treatment:
- #3: the left hilar lesion was reduced from 93mm to 51mm, which was reduced by 45.2%, the prevascular interstitial lymph nodes was reduced from 21mm to 8mm, which was reduced by 61.9%, and the subluminal lymph nodes were reduced from 24mm to 14mm, which was reduced by 41.7%. The overall best efficacy was assessed to achieve PR.
- #4: Lesion of the lower lobe of the left lung was reduced from 38mm to 29mm, a reduction of 23.7%. The overall best efficacy was assessed by SD.
- #5: The target lesion was located at the left base of the skull and stabilized at 45.5mm from 45.4mm. The overall best efficacy was assessed by SD.
- #6: the lesion of the lower lobe of the left lung was reduced from 67mm to 35mm, which was reduced by 47.8%. Left upper lobe lesions were shrunk from 19mm to 8mm, 57.9%, peritoneal stem lymph nodes were shrunk from 20mm to 6mm, 70%, paraaaortic lymph nodes were shrunk from 19mm to 7mm, 63.1%. The overall best efficacy assessment achieved confirmed PR.
- After 6 weeks of treatment:
- #7: the target lesion was located in the left hilar and lower lobe of the left lung. The left hilar lesion was reduced from 30.1mm to 24mm, which was 20.2%, and the lower lobe lesion was reduced from 18mm to 16mm, which was 11.1%. The overall best efficacy was assessed by SD.
- #8: the target lesion was located in the right liver, and the right liver lesion was reduced from 16mm to 11mm, which was reduced by 31.2%. The overall best efficacy was assessed to achieve PR.
The anti-B7H3 drug-antibody conjugate appears to have an acceptable safety profile and a promising efficacy in patients with advanced solid tumors
The Next Inflection Point for YL201: Converting Phase 1/1b Momentum Into Registrational Value
Roche’s $570M near-term commitment to MediLink’s YL201 is emblematic of where the ADC field is heading: value is being set at the intersection of differentiated biology, credible early clinical signals, and the ability to protect—and prosecute—platform and asset-level intellectual property. YL201’s Phase 1/1b dataset suggests meaningful activity across multiple hard-to-treat solid tumors (including ES-SCLC and NPC) with a manageable safety profile at recommended expansion doses, and the initiation of registrational Phase 3 trials indicates clear development ambition. In parallel, MediLink’s emerging patent footprint, still largely pending and geographically concentrated, highlights why IP maturation and freedom-to-operate monitoring will remain decisive as the program advances and partnering interest intensifies. With multiple ongoing studies yet to report results and combination strategies already moving into the clinic, the next 12–24 months will be critical to determine whether YL201 can translate early momentum into registrational outcomes—and whether its IP strategy can sustain competitive defensibility in the increasingly crowded B7-H3 ADC landscape.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About the author
Fabienne Massa, PhD., works for KnowMade in the field of Biotechnology and Life Sciences. She holds a PhD in Molecular and Cellular Biology from the IPMC (Sophia Antipolis, France). She also holds a Master of Business Management from IAE (Nice, France) and she previously worked in the pharmaceutical industry.
About KnowMade
KnowMade is a technology intelligence and IP strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patent information and scientific literature into actionable insights, providing high added value reports for decision makers working in R&D, innovation strategy, intellectual property, and marketing. Our experts provide prior art search, patent landscape analysis, freedom-to-operate analysis, IP due diligence, and monitoring services.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Sensors, Semiconductor Packaging, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
