
Are microneedles mature enough to replace hypodermic needles? Who are the top players and who is best placed in the race against COVID-19?
Publication August 2020
| Download Flyer | Download Sample |
Report’s Key Features

- PDF with > 140 slides
- Excel file > 2,450 patent families
- IP trends, including time-evolution of published patents, and countries of patent filings
- Patents’ legal status
- Ranking of main patent assignees
- Key players’ IP position and relative strength of their patent portfolios
- Summary of the IP related to applications: Cancer therapy, Cosmetic, Diabetes, Ophthalmic, Pain management and Vaccine.
- Summary of the IP related to technologies: Applicators, housing, Coated, Hollow, Porous, Soluble, Hydrogel and Solid microneedles.
- Analysis of patent oppositions (Europe) and review of key patents.
- Excel database containing all patents analyzed in the report, including applications and technologies segmentations
Discover more patent landscapes on healthcare. Unlock the full potential of your IP with our customized studies and comprehensive patent landscape analysis.
Microneedles for drug delivery, a new way to deliver drugs through the stratum corneum
The global drug delivery market has been observed to be as large as USD 440.5 billion in 2018. Hypodermic needles are known to be very efficient and they have been used immensely in the delivery of biological macromolecules, insulin, vaccines, etc. However, their invasive nature, stability issues, and non-compliance have always been the issues of concern and led to the requirement of highly skilled medical practitioners for administration.
In this context, microneedles have shown immense potential, since they can easily, painlessly and safely deliver drugs without special storage infrastructure. Hence, they can be self-administered by anybody, thereby eliminating the need for a clinic visit and the assistance of skilled medical practitioners. This serves as an important advantage, especially in the emerging markets. Moreover, microneedles do not create sharp waste, thereby eliminating the problem of discarding needles and hazardous risks. Patients suffering from needle phobia can easily use microneedles. Indeed, according to a study published in Behavioral Neurology, 2014, around 3-4% of the world population faces needle phobia.
Microneedles for drug delivery are attracting interest from big companies and start-ups alike and show immense potential that could transform the global transdermal market. Furthermore, in the actual race to find a COVID-19 vaccine, numerous companies (e.g. Kindeva (3M), Debiotech, Verndari, NanoPass, Bioserentach, Sorrento Therapeutics, Merck, etc.) have developed or shared their microneedle delivery system and promising results have already been released. Other fields, like cosmetic, ophthalmic and diabetes showed important technological progress in the past few years that could disrupt the existing market and replace the current standard drug delivery system.
In this evolving context, it is crucial to understand the intellectual property position and strategy of these different players. Such knowledge can help detect business risks and opportunities, anticipate emerging technologies, and enable strategic decisions to strengthen one’s market position.
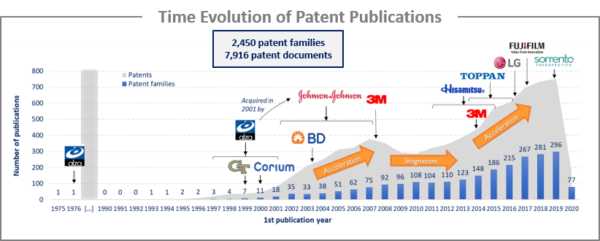
The analysis of the time evolution of patent publications shows that using microneedles for drug delivery were first filed in the 1971 by ALZA corporation patent (US3964482). However, it was not possible to make such microstructure devices until the 1990s, with the advent of high-precision microelectronics industrial tools. Hence, no patent families were published until 1993 and publications increased from 2001/2002, mainly due to the patenting activity of ALZA Corporation and Becton and Dickinson. A stagnation phase was observed between 2007 and 2012, followed by a second acceleration phase related to the patenting activities of 3M, Toppan Printing and Hisamistu Pharmaceutical. Patent publications then accelerated from 2015 with the entry of numerous newcomers and the expansion of the cosmetic and soluble microneedles field. The main patent assignees are Asian industrial players that develop an international intellectual property strategy. Asia (CN, JP and KR) and US are the main territories of protection, which appear to be strategic territories for these applicants.
Analysis by segment
 Microneedles for drug delivery can be applied to different fields and use different technologies (i.e. type of microneedles). Therefore, a technological segmentation was made for this IP landscape, as follows:
Microneedles for drug delivery can be applied to different fields and use different technologies (i.e. type of microneedles). Therefore, a technological segmentation was made for this IP landscape, as follows:
Applications
- Cancer therapy: microneedle patches present advantages in terms of controllability, easy applicability and predominant synergistic effect.
- Cosmetic: microneedles make it possible to enhance skin permeation to deliver cosmeceuticals.
- Diabetes: painless delivery of insulin transdermally into the stratum corneum through the miniature pores created by the microneedles.
- Ophthalmic: microneedles make it possible to deliver drug formulations to the target ocular tissues with great precision.
- Pain management: Injecting a vaccine strategically into the skin through microneedles can produce a significantly more intense immune response.
Technologies
- Applicators, housing & others: a key feature is the reliability that is required for applicators for consistent application/penetration of the microneedles.
- Coated microneedles: the coated drug formulation of the microneedle dissolves and deposits in the skin.
- Hollow microneedles: they make it possible to deliver continuously a large amount of drugs through the inserted hollow needles.
- Porous microneedles: they are a single-unit-drug delivery system, containing either a liquid or a dry drug formulation.
- Soluble microneedles: they dissolve upon contact with skin interstitial fluid and release the drug over time.
- Hydrogel microneedles: Once inserted, hydrogel microneedles rapidly take up interstitial fluid from the tissue, thus inducing the diffusion of the drug.
- Solid microneedles: they are creating transient aqueous microchannels through which the drug can be delivered through the stratum corneum.
Identifying the companies that have recently emerged in the IP landscape
Among the players that have filed patents related to microneedles for drug delivery, over 40 newcomers were identified. These companies are established companies and start-up firms developing their first products in the microneedle sector. Numerous IP newcomers are based in Asia and in the US with only a few in Europe. It is possible that one of these innovative companies could become one of the next healthcare unicorns that the big corporations will be tempted to acquire.
Key patent analysis
This IP study includes the selection and description of key patents. The key patent analysis includes the legal state of the family for each of the main territories, the number of received citations, the review of the main claim(s), the description of interesting features about the innovation disclosures and relevant figures illustrating how the innovation works. The description also contains information about the fact that the patent family was involved in patent litigation in the USA.

Moreover, the report also includes an Excel database with the >2450 patent families analyzed in this study. This useful patent database allows for multi-criteria searches and includes patent publication numbers, hyperlinks to the original documents, priority dates, titles, abstracts, patent assignees, each patent’s current legal status and segmentation to which it belongs.
Companies mentioned in this report
JOHNSON & JOHNSON; 3M; BECTON DICKINSON; HISAMITSU PHARMACEUTICAL; CORIUM; TOPPAN PRINTING; ZEALAND PHARMA; PROCTER & GAMBLE; COSMED PHARMACEUTICAL; FUJIFILM; NANOPASS TECHNOLOGY; BIOSERENTACH; SORRENTO THERAPEUTICS; THERAJECT; KIMBERLY CLARK; SILICON MICRODEVICES; ALTEA; NITTO; RAPHAS; ZOSANO PHARMA; BOEHRINGER INGELHEIM; NISSHA; VAXXAS; NANOMED; CLEARSIDE BIOMEDICAL; LG HOUSEHOLD & HEALTH CARE; LTS LOHMANN THERAPY SYSTEMS (non-exhaustive list)
